Biogenesis of mitochondrial β-barrel membrane proteins
- PMID: 39343721
- PMCID: PMC11452307
- DOI: 10.1002/2211-5463.13905
Biogenesis of mitochondrial β-barrel membrane proteins
Abstract
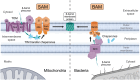
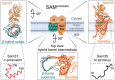
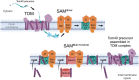
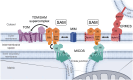
β-barrel membrane proteins in the mitochondrial outer membrane are crucial for mediating the metabolite exchange between the cytosol and the mitochondrial intermembrane space. In addition, the β-barrel membrane protein subunit Tom40 of the translocase of the outer membrane (TOM) is essential for the import of the vast majority of mitochondrial proteins encoded in the nucleus. The sorting and assembly machinery (SAM) in the outer membrane is required for the membrane insertion of mitochondrial β-barrel proteins. The core subunit Sam50, which has been conserved from bacteria to humans, is itself a β-barrel protein. The β-strands of β-barrel precursor proteins are assembled at the Sam50 lateral gate forming a Sam50-preprotein hybrid barrel. The assembled precursor β-barrel is finally released into the outer mitochondrial membrane by displacement of the nascent β-barrel, termed the β-barrel switching mechanism. SAM forms supercomplexes with TOM and forms a mitochondrial outer-to-inner membrane contact site with the mitochondrial contact site and cristae organizing system (MICOS) of the inner membrane. SAM shares subunits with the ER-mitochondria encounter structure (ERMES), which forms a membrane contact site between the mitochondrial outer membrane and the endoplasmic reticulum. Therefore, β-barrel membrane protein biogenesis is closely connected to general mitochondrial protein and lipid biogenesis and plays a central role in mitochondrial maintenance.
Keywords: Mco6; Mdm10; SAM; Sam35; Sam37; Sam50; mitochondria; outer membrane; sorting and assembly machinery; β‐barrel protein.
© 2024 The Author(s). FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Sam37 is crucial for formation of the mitochondrial TOM-SAM supercomplex, thereby promoting β-barrel biogenesis.J Cell Biol. 2015 Sep 28;210(7):1047-54. doi: 10.1083/jcb.201504119. J Cell Biol. 2015. PMID: 26416958 Free PMC article.
-
Role of the small protein Mco6 in the mitochondrial sorting and assembly machinery.Cell Rep. 2024 Mar 26;43(3):113805. doi: 10.1016/j.celrep.2024.113805. Epub 2024 Feb 19. Cell Rep. 2024. PMID: 38377000
-
Biogenesis of mitochondria: dual role of Tom7 in modulating assembly of the preprotein translocase of the outer membrane.J Mol Biol. 2011 Jan 7;405(1):113-24. doi: 10.1016/j.jmb.2010.11.002. Epub 2010 Nov 6. J Mol Biol. 2011. PMID: 21059357
-
Assembly of β-barrel proteins in the mitochondrial outer membrane.Biochim Biophys Acta. 2015 Jan;1853(1):74-88. doi: 10.1016/j.bbamcr.2014.10.006. Epub 2014 Oct 8. Biochim Biophys Acta. 2015. PMID: 25305573 Review.
-
Connection of Protein Transport and Organelle Contact Sites in Mitochondria.J Mol Biol. 2017 Jul 7;429(14):2148-2160. doi: 10.1016/j.jmb.2017.05.023. Epub 2017 May 30. J Mol Biol. 2017. PMID: 28576471 Review.
Cited by
-
The mitochondrial intermembrane space - a permanently proteostasis-challenged compartment.Biol Chem. 2025 May 27;406(5-7):263-294. doi: 10.1515/hsz-2025-0108. Print 2025 Aug 26. Biol Chem. 2025. PMID: 40435180 Review.
-
Mitochondria: the beating heart of the eukaryotic cell.FEBS Open Bio. 2024 Oct;14(10):1588-1590. doi: 10.1002/2211-5463.13884. FEBS Open Bio. 2024. PMID: 39367527 Free PMC article.
-
Molecular machineries and pathways of mitochondrial protein transport.Nat Rev Mol Cell Biol. 2025 Jul 3. doi: 10.1038/s41580-025-00865-w. Online ahead of print. Nat Rev Mol Cell Biol. 2025. PMID: 40610778 Review.
-
Mitochondrial Sorting and Assembly Machinery: Chaperoning a Moonlighting Role?Biochemistry. 2025 Jan 21;64(2):312-328. doi: 10.1021/acs.biochem.4c00727. Epub 2025 Jan 4. Biochemistry. 2025. PMID: 39754567 Free PMC article. Review.
References
-
- Roumia AF, Theodoropoulou MC, Tsirigos KD, Nielsen H and Bagos PG (2020) Landscape of eukaryotic transmembrane beta barrel proteins. J Proteome Res 19, 1209–1221. - PubMed
-
- Araiso Y, Imai K and Endo T (2022) Role of the TOM complex in protein import into mitochondria: structural views. Annu Rev Biochem 91, 679–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

