ACSL4-mediated lipid rafts prevent membrane rupture and inhibit immunogenic cell death in melanoma
- PMID: 39343834
- PMCID: PMC11439949
- DOI: 10.1038/s41419-024-07098-3
ACSL4-mediated lipid rafts prevent membrane rupture and inhibit immunogenic cell death in melanoma
Abstract
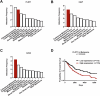
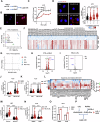
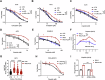
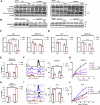
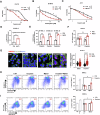
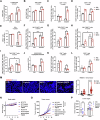
Chemotherapy including platinum-based drugs are a possible strategy to enhance the immune response in advanced melanoma patients who are resistant to immune checkpoint blockade (ICB) therapy. However, the immune-boosting effects of these drugs are a subject of controversy, and their impact on the tumor microenvironment are poorly understood. In this study, we discovered that lipid peroxidation (LPO) promotes the formation of lipid rafts in the membrane, which mediated by Acyl-CoA Synthetase Long Chain Family Member 4 (ACSL4) impairs the sensitivity of melanoma cells to platinum-based drugs. This reduction primarily occurs through the inhibition of immunogenic ferroptosis and pyroptosis by reducing cell membrane pore formation. By disrupting ACSL4-mediaged lipid rafts via the removal of membrane cholesterol, we promoted immunogenic cell death, transformed the immunosuppressive environment, and improved the antitumor effectiveness of platinum-based drugs and immune response. This disruption also helped reverse the decrease in CD8+ T cells while maintaining their ability to secrete cytokines. Our results reveal that ACSL4-dependent LPO is a key regulator of lipid rafts formation and antitumor immunity, and that disrupting lipid rafts has the potential to enhance platinum-based drug-induced immunogenic ferroptosis and pyroptosis in melanoma. This novel strategy may augment the antitumor immunity of platinum-based therapy and further complement ICB therapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Pires da Silva I, Ahmed T, Reijers ILM, Weppler AM, Betof Warner A, Patrinely JR, et al. Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: a multicentre, retrospective, cohort study. Lancet Oncol. 2021;22:836–47. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

