HECTD2 as a target for veratric acid in the regulation of ferroptosis in renal cell carcinoma
- PMID: 39343853
- PMCID: PMC11439856
- DOI: 10.1007/s00726-024-03419-0
HECTD2 as a target for veratric acid in the regulation of ferroptosis in renal cell carcinoma
Abstract
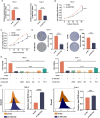
Function of HECTD2 in renal cell carcinoma malignant progression is undefined. Molecular mechanism behind anti-cancer effects of veratric acid (VA) from traditional Chinese medicine (TCM) is underexplored. The Cancer Genome Atlas was leveraged to study HECTD2 expression in renal cell carcinoma and its relationship with histological grading. Kaplan-Meier survival analysis was performed. HECTD2 expression was detected in cancer cells and tissues via qRT-PCR and immunohistochemistry. GPX4 and SLC7A11 expression in tumor samples with high or low HECTD2 expression was examined by immunohistochemistry, cell viability by CCK8, cell proliferation by colony formation assay, lipid ROS and mitochondrial superoxide levels by flow cytometry, Fe2+ and MDA content by assay kits, and GPX4 and SLC7A11 proteins by western blot. SeeSAR software screened TCM small molecule compounds with highest affinity to HECTD2, confirmed with cellular thermal shift assay. VA IC50 was measured by CCK8. Xenograft model was developed and treated with VA. Tumor size and weight were monitored, with immunohistochemistry to detect HECTD2 expression in tumors and assess ferroptosis-related markers. HECTD2 was overexpressed in tumor tissues and cells, which positively correlated with histological grading. HECTD2 depletion inhibited cell vitality and proliferation, raised intracellular lipid ROS, mitochondrial superoxide, Fe2+, and MDA. HECTD2 was a target with highest VA affinity. In vitro and vivo experiments concurred that VA treatment hindered malignancy of renal cell carcinoma and enhanced its susceptibility to ferroptosis. HECTD2 supports ferroptosis resistance in renal cell carcinoma, but VA, through its targeting of HECTD2, initiates ferroptosis, showcasing its anti-cancer efficacy.
Keywords: Ferroptosis; HECTD2; Renal cell carcinoma; Traditional Chinese medicine; Veratric acid.
© 2024. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures





References
-
- Bahadoram S, Davoodi M, Hassanzadeh S, Bahadoram M, Barahman M, Mafakher L (2022) Renal cell carcinoma: an overview of the epidemiology, diagnosis, and treatment. G Ital Nefrol. 39:2022 - PubMed
MeSH terms
Substances
Grants and funding
- 2023SZZ004/Science and Technology Bureau of Deyang City, Project Funding of Social Development
- 2023SZZ004/Science and Technology Bureau of Deyang City, Project Funding of Social Development
- FHS202301/Deyang People's Hospital 2023 Hospital Incubation Project
- ZQY2023ZX02/Sichuan Provincial Key Chinese Culture Research Institute - Tibetan and Qiang Yi Culture Research Institute Key Project Funding
LinkOut - more resources
Full Text Sources
Medical

