Introducing the Biosimilar Paradigm to Neurology: The Totality of Evidence for the First Biosimilar Natalizumab
- PMID: 39343860
- PMCID: PMC11530514
- DOI: 10.1007/s40259-024-00671-4
Introducing the Biosimilar Paradigm to Neurology: The Totality of Evidence for the First Biosimilar Natalizumab
Abstract
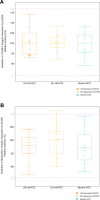
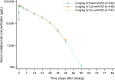
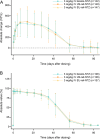
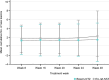
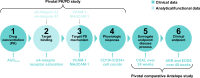
A biosimilar medicine is a successor to a reference ('originator'/'original-brand') biologic medicine brought to market once the patent and exclusive marketing rights for the reference have expired. Biosimilar natalizumab (PB006 [biosim-NTZ]; developed by Polpharma Biologics S.A. and marketed globally as Tyruko®; Sandoz) has been developed as a successor to reference natalizumab (Tysabri® [ref-NTZ]; Biogen) and is the first US Food and Drug Administration (FDA)-approved and European Medicines Agency (EMA)-approved biosimilar in neurology. As per the FDA and EMA indications for ref-NTZ, biosim-NTZ is approved to treat relapsing forms of multiple sclerosis (USA, EU) and Crohn's disease (USA only). Approval of biosim-NTZ was based on the 'totality of evidence', a comprehensive body of data collected during the development process, demonstrating similarity to its reference medicine. The foundational step of demonstrating structural and functional similarity between biosim-NTZ and ref-NTZ confirmed identical primary and indistinguishable higher order structures, as well as matching binding affinity to α4β1/α4β7 integrins. Following the confirmation of matching structure and function, pharmacokinetic/pharmacodynamic similarity of biosim-NTZ to ref-NTZ in healthy subjects was demonstrated, with no clinically meaningful differences identified in safety and immunogenicity. A comparative, double-blind, randomized study (Antelope) was also conducted in patients with relapsing-remitting multiple sclerosis and demonstrated matching efficacy, safety, and immunogenicity with no clinically meaningful differences between biosim-NTZ and ref-NTZ. This review presents the totality of evidence that confirmed the biosimilarity of biosimilar natalizumab to its reference medicine, which supported its approval by the FDA and the EMA. [Graphical plain language summary available].
© 2024. The Author(s).
Conflict of interest statement
Krzysztof Selmaj, Karsten Roth, Josef Höfler, Klaus Vitzithum, Rafał Derlacz, Oliver von Richter, Cyrill Hornuss, Johann Poetzl, Barry Singer, and Laura Jacobs. Krzysztof Selmaj has received honoraria for speaking, consulting, and serving on advisory boards for Merck, Novartis, Roche, Biogen, Celgene, and TG Therapeutics. Karsten Roth has received personal compensation for serving as an employee of Polpharma Biologics S.A. Klaus Vitzithum has received personal compensation for serving as an employee of Polpharma Biologics S.A. Josef Höfler has received personal compensation for serving as an employee of Staburo GmbH. Rafał Derlacz has received personal compensation for serving as an employee of Polpharma Biologics S.A. Oliver von Richter has received personal compensation for serving as an employee of Hexal AG (a Sandoz company). Cyrill Hornuss has received personal compensation for serving as an employee of Hexal AG (a Sandoz company). Johann Poetzl has received personal compensation for serving as an employee of Hexal AG (a Sandoz company). Laura Jacobs has received personal compensation for serving as an employee of Hexal AG (a Sandoz company). Barry Singer has received research grant support from AbbVie, Biogen, Bristol Myers Squibb, Greenwich Biosciences, Novartis, Sanofi, and TG Therapeutics, and consulting and/or speaking fees from Alexion, Biogen, Bristol Myers Squibb, Cigna, Cycle, EMD Serono, Genentech, Horizon, Janssen, Novartis, Octave Bioscience, Roche, Sanofi, Sandoz, and TG Therapeutics.
Figures

Similar articles
-
Comparative immunogenicity assessment of biosimilar natalizumab to its reference medicine: a matching immunogenicity profile.Front Immunol. 2024 Dec 19;15:1414304. doi: 10.3389/fimmu.2024.1414304. eCollection 2024. Front Immunol. 2024. PMID: 39749348 Free PMC article. Clinical Trial.
-
Pharmacokinetic and pharmacodynamic similarity of biosimilar natalizumab (PB006) to its reference medicine: a randomized controlled trial.Expert Opin Biol Ther. 2023 Jul-Dec;23(12):1287-1297. doi: 10.1080/14712598.2023.2290530. Epub 2023 Dec 28. Expert Opin Biol Ther. 2023. PMID: 38044885 Clinical Trial.
-
Efficacy and Safety of Proposed Biosimilar Natalizumab (PB006) in Patients With Relapsing-Remitting Multiple Sclerosis: The Antelope Phase 3 Randomized Clinical Trial.JAMA Neurol. 2023 Mar 1;80(3):298-307. doi: 10.1001/jamaneurol.2022.5007. JAMA Neurol. 2023. PMID: 36689214 Free PMC article. Clinical Trial.
-
'Totality of Evidence' Approach in the Development of GP2017, an Approved Adalimumab Biosimilar.Adv Ther. 2024 May;41(5):1795-1814. doi: 10.1007/s12325-024-02809-w. Epub 2024 Mar 21. Adv Ther. 2024. PMID: 38514505 Free PMC article. Review.
-
PB006: A Natalizumab Biosimilar.Clin Drug Investig. 2024 May;44(5):367-370. doi: 10.1007/s40261-024-01360-4. Epub 2024 Apr 29. Clin Drug Investig. 2024. PMID: 38683493 Free PMC article. Review.
Cited by
-
Comparative immunogenicity assessment of biosimilar natalizumab to its reference medicine: a matching immunogenicity profile.Front Immunol. 2024 Dec 19;15:1414304. doi: 10.3389/fimmu.2024.1414304. eCollection 2024. Front Immunol. 2024. PMID: 39749348 Free PMC article. Clinical Trial.
-
Comparing STRATIFY JCV™ DxSelect™ and IMMUNOWELL™ JCV Tests in RRMS to Assess PML Risk.Curr Neuropharmacol. 2025;23(10):1294-1300. doi: 10.2174/011570159X372688250226110925. Curr Neuropharmacol. 2025. PMID: 40197186
References
-
- McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA. 2021;325:765–79. - PubMed
-
- Brownlee WJ, Wolf C, Hartung HP, et al. Use of follow-on disease-modifying treatments for multiple sclerosis: consensus recommendations. Mult Scler. 2022;28:2177–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

