Differences in motor learning-related structural plasticity of layer 2/3 parvalbumin-positive interneurons of the young and aged motor cortex
- PMID: 39343864
- PMCID: PMC12181450
- DOI: 10.1007/s11357-024-01350-6
Differences in motor learning-related structural plasticity of layer 2/3 parvalbumin-positive interneurons of the young and aged motor cortex
Abstract
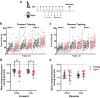
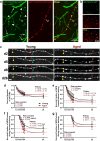
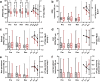
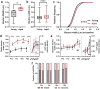
Changes to neuronal connectivity are believed to be a key factor in cognitive impairments associated with normal aging. Because of its effect on activities of daily living, deficient motor control is a critical type of cognitive decline to understand. Diminished inhibitory networks in the cortex are implicated in such motor control deficits, pointing to the connectivity of inhibitory cortical interneurons as an important area for study. Here, we used chronic two-photon microscopy to track the structural plasticity of en passant boutons (EPBs) of parvalbumin-positive interneurons in the mouse motor cortex in the first longitudinal, in vivo study of inhibitory interneuron synapses in the context of aging. Young (3-5 months) and aged (23-28 months) mice underwent training on the accelerating rotarod to evoke motor learning-induced structural plasticity. Our analysis reveals that, in comparison with axons from young mice, those from aged mice have fewer EPBs at baseline that also tend to be larger in size. Aged axons also express learning-related structural plasticity-like new bouton stabilization and bouton enlargement-that is less persistent than that of young axons. This study reveals striking baseline differences in young and aged axon morphology as well as differences in the deployment of learning-related structural plasticity across axons.
Keywords: Aging; Axon; Cortex; Interneuron; Motor learning; Structural plasticity.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing financial interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

