Preventive effect of probiotics on oral mucositis induced by anticancer therapy: a systematic review and meta-analysis of randomized controlled trials
- PMID: 39343876
- PMCID: PMC11441129
- DOI: 10.1186/s12903-024-04955-7
Preventive effect of probiotics on oral mucositis induced by anticancer therapy: a systematic review and meta-analysis of randomized controlled trials
Abstract
Background: Oral mucositis (OM) is a prevalent and painful complication in patients undergoing anticancer treatment, which significantly impacts patients' quality of life (QoL) and adherence to therapy. The use of oral probiotics as a preventive strategy for OM has shown promise, but the clinical evidence remains inconclusive. This meta-analysis of randomized controlled trials (RCTs) aims to evaluate the efficacy of probiotics in preventing OM caused by radiotherapy and/or chemotherapy.
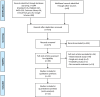
Methods: A comprehensive search of PubMed, EMBASE, Web of Science, Cochrane Library, and ClinicalTrials.gov was conducted up to January 31, 2024, to identify eligible RCTs. The primary outcomes were the incidences of severe OM and all-grade OM. Secondary outcomes included rates of anticancer treatment completion, clinical response, requirement for enteral nutrition, time course of OM, body weight loss, QoL, and adverse events (AEs). Risk ratios (RRs) with 95% confidence intervals (CIs) were calculated.
Results: A total of 12 RCTs involving 1,376 patients were included in the quantitative analysis. Probiotics administration significantly reduced the risk of severe OM (RR = 0.61, 95%CI: 0.53-0.72, P < 0.001) and all-grade OM (RR = 0.90, 95%CI: 0.82-0.98, P = 0.016) compared to the control group. Multi-strain probiotics formulations were more effective than single-strain probiotics in preventing severe OM (P = 0.011). There were no significant differences between the probiotics and control groups regarding anticancer treatment completion (RR = 1.03, 95%CI: 0.98-1.08, P = 0.198), clinical response to therapy (RR = 1.05, 95%CI: 0.94-1.17, P = 0.406), or the need for enteral nutrition (RR = 1.28, 95%CI: 0.49-3.35, P = 0.680). AEs related to probiotics were rare, with no serious AEs attributable to probiotics use.
Conclusions: Oral probiotics are both safe and effective in preventing and reducing the severity of OM in patients undergoing anticancer therapy. Multi-strain probiotics demonstrate superior efficacy compared to single-strain probiotics. Further research is warranted to confirm these findings and optimize probiotic treatment strategies for cancer patients.
Keywords: Chemotherapy; Meta-analysis; Oral mucositis; Probiotics; Radiotherapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Bowen JM, Wardill HR. Advances in the understanding and management of mucositis during stem cell transplantation. Curr Opin Support Palliat Care. 2017;11:341–6. 10.1097/SPC.0000000000000310. - PubMed
-
- Silverman S. Jr. Diagnosis and management of oral mucositis. J Support Oncol. 2007;5:13–21. - PubMed
-
- Sonis ST, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100:1995–2025. 10.1002/cncr.20162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

