Histone deacetylase inhibition enhances extracellular vesicles from muscle to promote osteogenesis via miR-873-3p
- PMID: 39343927
- PMCID: PMC11439940
- DOI: 10.1038/s41392-024-01976-0
Histone deacetylase inhibition enhances extracellular vesicles from muscle to promote osteogenesis via miR-873-3p
Abstract
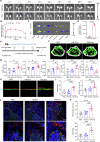
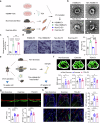
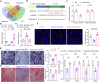
Regular physical activity is widely recognized for reducing the risk of various disorders, with skeletal muscles playing a key role by releasing biomolecules that benefit multiple organs and tissues. However, many individuals, particularly the elderly and those with clinical conditions, are unable to engage in physical exercise, necessitating alternative strategies to stimulate muscle cells to secrete beneficial biomolecules. Histone acetylation and deacetylation significantly influence exercise-induced gene expression, suggesting that targeting histone deacetylases (HDACs) could mimic some exercise responses. In this study, we explored the effects of the HDAC inhibitor Trichostatin A (TSA) on human skeletal muscle myoblasts (HSMMs). Our findings showed that TSA-induced hyperacetylation enhanced myotube fusion and increased the secretion of extracellular vesicles (EVs) enriched with miR-873-3p. These TSA-EVs promoted osteogenic differentiation in human bone marrow mesenchymal stem cells (hBMSCs) by targeting H2 calponin (CNN2). In vivo, systemic administration of TSA-EVs to osteoporosis mice resulted in significant improvements in bone mass. Moreover, TSA-EVs mimicked the osteogenic benefits of exercise-induced EVs, suggesting that HDAC inhibition can replicate exercise-induced bone health benefits. These results demonstrate the potential of TSA-induced muscle-derived EVs as a therapeutic strategy to enhance bone formation and prevent osteoporosis, particularly for individuals unable to exercise. Given the FDA-approved status of various HDAC inhibitors, this approach holds significant promise for rapid clinical translation in osteoporosis treatment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Watson, M. C. & Lloyd, J. Physical activity: manifold benefits for health and wellbeing. BMJ376, o815 (2022). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

