Dynamic glycolytic reprogramming effects on dendritic cells in pancreatic ductal adenocarcinoma
- PMID: 39343933
- PMCID: PMC11441259
- DOI: 10.1186/s13046-024-03192-8
Dynamic glycolytic reprogramming effects on dendritic cells in pancreatic ductal adenocarcinoma
Abstract
Background: Pancreatic ductal adenocarcinoma tumors exhibit resistance to chemotherapy, targeted therapies, and even immunotherapy. Dendritic cells use glucose to support their effector functions and play a key role in anti-tumor immunity by promoting cytotoxic CD8+ T cell activity. However, the effects of glucose and lactate levels on dendritic cells in pancreatic ductal adenocarcinoma are unclear. In this study, we aimed to clarify how glucose and lactate can impact the dendritic cell antigen-presenting function and elucidate the relevant mechanisms.
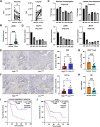
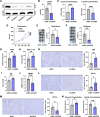
Methods: Glycolytic activity and immune cell infiltration in pancreatic ductal adenocarcinoma were evaluated using patient-derived organoids and resected specimens. Cell lines with increased or decreased glycolysis were established from KPC mice. Flow cytometry and single-cell RNA sequencing were used to evaluate the impacts on the tumor microenvironment. The effects of glucose and lactate on the bone marrow-derived dendritic cell antigen-presenting function were detected by flow cytometry.
Results: The pancreatic ductal adenocarcinoma tumor microenvironment exhibited low glucose and high lactate concentrations from varying levels of glycolytic activity in cancer cells. In mouse transplantation models, tumors with increased glycolysis showed enhanced myeloid-derived suppressor cell infiltration and reduced dendritic cell and CD8+ T cell infiltration, whereas tumors with decreased glycolysis displayed the opposite trends. In three-dimensional co-culture, increased glycolysis in cancer cells suppressed the antigen-presenting function of bone marrow-derived dendritic cells. In addition, low-glucose and high-lactate media inhibited the antigen-presenting and mitochondrial functions of bone marrow-derived dendritic cells.
Conclusions: Our study demonstrates the impact of dynamic glycolytic reprogramming on the composition of immune cells in the tumor microenvironment of pancreatic ductal adenocarcinoma, especially on the antigen-presenting function of dendritic cells.
Keywords: Antigen-presenting function; Dendritic cell; Glycolysis; Pancreatic ductal adenocarcinoma; Tumor microenvironment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

