MRI detection of free-contrast agent nanoparticles
- PMID: 39344270
- PMCID: PMC11604830
- DOI: 10.1002/mrm.30292
MRI detection of free-contrast agent nanoparticles
Abstract
Purpose: The integration of nanotechnology into biomedical imaging has significantly advanced diagnostic and theranostic capabilities. However, nanoparticle detection in imaging relies on functionalization with appropriate probes. In this work, a new approach to visualize free-label nanoparticles using MRI and MRS techniques is described, consisting of detecting by 1H CSI specific proton signals belonging to the components naturally present in most of the nanosystems used in preclinical and clinical research.
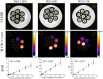
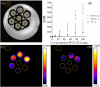
Methods: Three different nanosystems, namely lipid-based micelles, liposomes, and perfluorocarbon-based nanoemulsions, were synthesized, characterized by high resolution NMR and then visualized by 1H CSI at 300 MHz. Subsequently the best 1H CSI performing system was administered to murine models of cancer to evaluate the possibility of tracking the nanosystem by looking at its proton associated signal. Furthermore, an in vitro comparison between 1H CSI and 19F MRI was carried out.
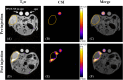
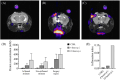
Results: The study successfully demonstrates the feasibility of detecting nanoparticles using MRI/MRS without probe functionalization, employing 1H CSI. Among the nanosystems tested, the perfluorocarbon-based nanoemulsion exhibited the highest SNR. Consequently, it was evaluated in vivo, where its detection was achievable within tumors and inflamed regions via 1H CSI, and in lymph nodes via PRESS.
Conclusions: These findings present a promising avenue for nanoparticle imaging in biomedical applications, offering potential enhancements to diagnostic and theranostic procedures. This non-invasive approach has the capacity to advance imaging techniques and expand the scope of nanoparticle-based biomedical research. Further exploration is necessary to fully explore the implications and applications of this method.
Keywords: CSI; MRI; liposomes; micelles; nanoemulsions; nanoparticles.
© 2024 The Author(s). Magnetic Resonance in Medicine published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Conflict of interest statement
The authors disclose the filing of an Italian patent entitled “Nanosystems comprising label‐free nanoparticles and uses thereof” concerning the topic of this manuscript (application date: 08/08/2023, N° 102023000016998).
Figures



References
-
- Chauhan G, Madou MJ, Kalra S, Chopra V, Ghosh D, Martinez‐Chapa SO. Nanotechnology for COVID‐19: therapeutics and vaccine research. ACS Nano. 2020;14:7760‐7782. - PubMed
-
- Crommelin DJA, van Hoogevest P, Storm G. The role of liposomes in clinical nanomedicine development. What now? Now what? J Control Release. 2020;318:256‐263. - PubMed
-
- Harshita BMA, Das SS, Pottoo FH, Beg S, Rahman Z. Lipid‐based Nanosystem As intelligent carriers for versatile drug delivery applications. Curr Pharm des. 2020;26:1167‐1180. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

