Predicting Individual Treatment Effects to Determine Duration of Dual Antiplatelet Therapy After Stent Implantation
- PMID: 39344653
- PMCID: PMC11681487
- DOI: 10.1161/JAHA.124.034862
Predicting Individual Treatment Effects to Determine Duration of Dual Antiplatelet Therapy After Stent Implantation
Abstract
Background: After coronary stent implantation, prolonged dual antiplatelet therapy (DAPT) increases bleeding risk, requiring personalization of DAPT duration. The aim of this study was to develop and validate a machine learning model to predict optimal DAPT duration after contemporary drug-eluting stent implantation in patients with coronary artery disease.
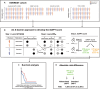
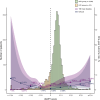
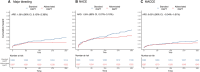
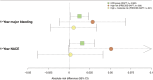
Methods and results: The One-Month DAPT, RESET (Real Safety and Efficacy of 3-Month Dual Antiplatelet Therapy Following Endeavor Zotarolimus-Eluting Stent Implantation), and IVUS-XPL (Impact of Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesion) trials provided a derivation cohort (n=6568). Using the X-learner approach, an individualized DAPT score was developed to determine the therapeutic benefit of abbreviated (1-6 months) versus standard (12-month) DAPT using various predictors. The primary outcome was major bleeding; the secondary outcomes included 1-year major adverse cardiac and cerebrovascular events and 1-year net adverse clinical events. The risk reduction with abbreviated DAPT (3 months) in the individualized DAPT-determined higher predicted benefit group was validated in the TICO (Ticagrelor Monotherapy After 3 Months in the Patients Treated With New Generation Sirolimus-Eluting Stent for Acute Coronary Syndrome) trial (n=3056), which enrolled patients with acute coronary syndrome treated with ticagrelor. The validation cohort comprised 1527 abbreviated and 1529 standard DAPT cases. Major bleeding occurred in 25 (1.7%) and 45 (3.0%) patients in the abbreviated and standard DAPT groups, respectively. The individualized DAPT score identified 2582 (84.5%) participants who would benefit from abbreviated DAPT, which was significantly associated with a lower major bleeding risk (absolute risk difference [ARD], 1.26 [95% CI, 0.15-2.36]) and net adverse clinical events (ARD, 1.59 [95% CI, 0.07-3.10]) but not major adverse cardiac and cerebrovascular events (ARD, 0.63 [95% CI, -0.34 to 1.61]), compared with standard DAPT in the higher predicted benefit group. Abbreviated DAPT had no significant difference in clinical outcomes of major bleeding (ARD, 1.49 [95% CI, -1.74 to 4.72]), net adverse clinical events (ARD, 2.57 [95% CI, -1.85 to 6.99]), or major adverse cardiac and cerebrovascular events (ARD, 1.54 [95% CI, -1.26 to 4.34]), compared with standard DAPT in the individualized DAPT-determined lower predicted benefit group.
Conclusions: Machine learning using the X-learner approach identifies patients with acute coronary syndrome who may benefit from abbreviated DAPT after drug-eluting stent implantation, laying the groundwork for personalized antiplatelet therapy.
Keywords: drug‐eluting stents; dual antiplatelet therapy; machine learning; percutaneous coronary intervention; treatment effect heterogeneity.
Figures
References
-
- Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation. 2016;134:e123–e155. doi: 10.1161/CIR.0000000000000404 - DOI - PubMed
-
- Valgimigli M, Bueno H, Byrne RA, Collet J‐P, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419 - DOI - PubMed
-
- D'Ascenzo F, Moretti C, Bianco M, Bernardi A, Taha S, Cerrato E, Omedè P, Montefusco A, Frangieh AH, Lee CW, et al. Meta‐analysis of the duration of dual antiplatelet therapy in patients treated with second‐generation drug‐eluting stents. Am J Cardiol. 2016;117:1714–1723. doi: 10.1016/j.amjcard.2016.03.005 - DOI - PubMed
-
- Bianco M, Careggio A, Destefanis P, Luciano A, Perrelli MG, Quadri G, Rossini R, Campo G, Vizzari G, D'Ascenzo F, et al. P2Y12 inhibitors monotherapy after short course of dual antiplatelet therapy in patients undergoing percutaneous coronary intervention: a meta‐analysis of randomized clinical trials including 29 089 patients. Eur Heart J—Cardiovasc Pharmacother. 2021;7:196–205. doi: 10.1093/ehjcvp/pvaa038 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

