Low Frequency Ventilation During Cardiopulmonary Bypass to Protect Postoperative Lung Function in Cardiac Valvular Surgery: The PROTECTION Phase II Randomized Trial
- PMID: 39344668
- PMCID: PMC11681471
- DOI: 10.1161/JAHA.124.035011
Low Frequency Ventilation During Cardiopulmonary Bypass to Protect Postoperative Lung Function in Cardiac Valvular Surgery: The PROTECTION Phase II Randomized Trial
Abstract
Background: Cardiac surgery with cardiopulmonary bypass (CPB) triggers pulmonary injury. In this trial we assessed the feasibility, safety, and efficacy of low frequency ventilation (LFV) during CPB in patients undergoing valvular surgery.
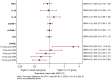
Methods and results: Patients with severe mitral or aortic valve disease were randomized to either LFV or usual care. Primary outcomes included release of generic inflammatory and vascular biomarkers and the lung-specific biomarker sRAGE (soluble receptor for advance glycation end products) up to 24 hours postsurgery. Secondary outcomes included pulmonary function tests and 6-minute walking test up to 8 weeks postdischarge. Sixty-three patients were randomized (33 LFV versus 30 usual care). Mean age was 66.8 years and 30% were female. LFV was associated with changes of sRAGE (soluble receptor for advance glycation end products) levels (geometric mean ratio, 3.05; [95% CI, 1.13-8.24] 10 minutes post CPB, and 1.07 [95% CI, 0.64-1.79], 0.84 [95% CI, 0.55-1.27], 0.67 [95% CI, 0.42-1.07], and 0.62 [95% CI, 0.45-0.85] at 2, 6, 12, and 24 hours post CPB respectively). No changes were observed for any of the generic biomarkers. Respiratory index soon after surgery (mean difference, -0.61 [95% CI, -1.24 to 0.015] 10 minutes post end of CPB), forced expiratory volume after 1 second/forced vital capacity ratio (0.050 [95% CI, 0.007-0.093] at 6 to 8 weeks pos-surgery), Forced vital capacity alone (95% CI, -0.191 L [-0.394 to 0.012]) and 6-minute walking test score at discharge (63.2 m [95% CI, 12.9-113.6]) were better preserved in the LFV group. No other differences were noted.
Conclusions: The use of LFV during CPB in patients undergoing valvular surgery was feasible and safe and was associated with changes in sRAGE levels along with better preserved lung function and walking performance. These observations warrant further investigation in larger future studies.
Registration: URL: https://www.isrctn.com; Unique Identifier: ISRCTN75795633.
Keywords: cardiopulmonary bypass; low frequency ventilation; lung protection; pulmonary function tests; sRAGE; valvular surgery.
Figures

References
-
- Sanfilippo F, Palumbo GJ, Bignami E, Pavesi M, Ranucci M, Scolletta S, Pelosi P, Astuto M. Acute respiratory distress syndrome in the perioperative period of cardiac surgery: predictors, diagnosis, prognosis, management options, and future directions. J Cardiothorac Vasc Anesth. 2022;36:1169–1179. doi: 10.1053/j.jvca.2021.04.024 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

