SARS-CoV-2 ORF3a induces COVID-19-associated kidney injury through HMGB1-mediated cytokine production
- PMID: 39345136
- PMCID: PMC11559048
- DOI: 10.1128/mbio.02308-24
SARS-CoV-2 ORF3a induces COVID-19-associated kidney injury through HMGB1-mediated cytokine production
Abstract
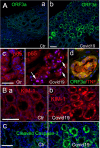
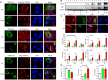
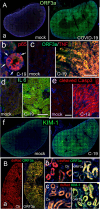
The primary challenge posed by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is COVID-19-related mortality, often exacerbated by additional medical complications, such as COVID-19-associated kidney injuries (CAKIs). Up to half of COVID-19 patients experience kidney complications, with those facing acute respiratory failure and kidney injury having the worst overall prognosis. Despite the significant impact of CAKI on COVID-19-related mortality and its enduring effects in long COVID, the underlying causes and molecular mechanisms of CAKI remain elusive. In this study, we identified a functional relationship between the expression of the SARS-CoV-2 ORF3a protein and inflammation-driven apoptotic death of renal tubular epithelial cells in patients with CAKI. We demonstrate in vitro that ORF3a independently induces renal cell-specific apoptotic cell death, as evidenced by the elevation of kidney injury molecule-1 (KIM-1) and the activation of NF-kB-mediated proinflammatory cytokine (TNFα and IL-6) production. By examining kidney tissues of SARS-CoV-2-infected K18-ACE2 transgenic mice, we observed a similar correlation between ORF3a-induced cytopathic changes and kidney injury. This correlation was further validated through reconstitution of the ORF3a effects via direct adenoviral injection into mouse kidneys. Through medicinal analysis, we identified a natural compound, glycyrrhizin (GL4419), which not only blocks viral replication in renal cells, but also mitigates ORF3a-induced renal cell death by inhibiting activation of a high mobility group box 1 (HMGB1) protein, leading to a reduction of KIM-1. Moreover, ORF3a interacts with HMGB1. Overproduction or downregulation of hmgb1 expression results in correlative changes in renal cellular KIM-1 response and respective cytokine production, implicating a crucial role of HMGB1 in ORF3a-inflicted kidney injuries. Our data suggest a direct functional link between ORF3a and kidney injury, highlighting ORF3a as a unique therapeutic target contributing to CAKI.
Importance: The major challenge of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during the pandemic is COVID-19-related mortality, which has tragically claimed millions of lives. COVID-19-associated morbidity and mortality are often exacerbated by pre-existing medical conditions, such as chronic kidney diseases (CKDs), or the development of acute kidney injury (AKI) due to COVID-19, collectively known as COVID-19-associated kidney injuries (CAKIs). Patients who experience acute respiratory failure with CAKI have the poorest clinical outcomes, including increased mortality. Despite these alarming clinical findings, there is a critical gap in our understanding of the underlying causes of CAKI. Our study establishes a direct correlation between the expression of the SARS-CoV-2 viral ORF3a protein and kidney injury induced by ORF3a linking to CAKI. This functional relationship was initially observed in our clinical studies of COVID-19 patients with AKI and was further validated through animal and in vitro cellular studies, either by expressing ORF3a alone or in the context of viral infection. By elucidating this functional relationship and its underlying mechanistic pathways, our research deepens the understanding of COVID-19-associated kidney diseases and presents potential therapeutic avenues to address the healthcare challenges faced by individuals with underlying conditions.
Keywords: CAKI; HMGB1; K18-hACE2 mice; NF-kB; ORF3a; SARS-CoV-2; TNFα and IL-6; glycyrrhizin; viral infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Klimkiewicz J, Grzywacz A, Michałowski A, Gutowski M, Paryż K, Jędrych E, Lubas A. 2024. Acute kidney injury and chronic kidney disease and their impacts on prognosis among patients with severe COVID-19 Pneumonia: an expert center case-cohort study. J Clin Med 13:1486. doi:10.3390/jcm13051486 - DOI - PMC - PubMed
-
- Portolés J, López-Sánchez P, Martin-Rodríguez L, Serrano-Salazar ML, Valdenebro-Recio M, Ramos A, Malo RM, Zalamea F, Martin-Giner JM, Marques M, Ortiz A. 2023. Acute and chronic kidney disease and risk of hospital mortality during COVID-19 pandemic waves in the pre-vaccination era. Clin Kidney J 16:374–383. doi:10.1093/ckj/sfac239 - DOI - PMC - PubMed
-
- Ahsan MN, Asghar MS, Iqbal S, Alvi H, Akram M, Fayyaz B, Irshad SG, Ullah I, Alvi S, Yousaf Z. 2023. Outcomes of COVID-19 patients with acute kidney injury and longitudinal analysis of laboratory markers during the hospital stay: a multi-center retrospective cohort experience from Pakistan. Medicine (Baltimore) 102:e32919. doi:10.1097/MD.0000000000032919 - DOI - PMC - PubMed
-
- Aklilu AM, Kumar S, Nugent J, Yamamoto Y, Coronel-Moreno C, Kadhim B, Faulkner SC, O’Connor KD, Yasmin F, Greenberg JH, Moledina DG, Testani JM, Wilson FP. 2024. COVID-19-associated acute kidney injury and longitudinal kidney outcomes. JAMA Intern Med 184:414–423. doi:10.1001/jamainternmed.2023.8225 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R01NS102589, R01NS105633/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- I01BX004652/U.S. Department of Veterans Affairs (VA)
- SC170199/U.S. Department of Defense (DOD)
- G12MD007597/HHS | NIH | National Institute on Minority Health and Health Disparities (NIMHD)
- I01 BX004652/BX/BLRD VA/United States
- SC1AI112785/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- I01RX003060 ,I01BX004652/U.S. Department of Veterans Affairs (VA)
- R01 NS107262/NS/NINDS NIH HHS/United States
- R01 NS102589/NS/NINDS NIH HHS/United States
- I01 RX003060/RX/RRD VA/United States
- G12 MD007597/MD/NIMHD NIH HHS/United States
- R01NS107262/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R21 AI175931/AI/NIAID NIH HHS/United States
- R01HL082517/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- U54 MD007597/MD/NIMHD NIH HHS/United States
- R01 NS105633/NS/NINDS NIH HHS/United States
- R01 HL082517/HL/NHLBI NIH HHS/United States
- R21AI175931/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- SC1 AI112785/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

