Duck circovirus regulates the expression of duck CLDN2 protein by activating the MAPK-ERK pathway to affect its adhesion and infection
- PMID: 39345142
- PMCID: PMC11495148
- DOI: 10.1128/jvi.00497-24
Duck circovirus regulates the expression of duck CLDN2 protein by activating the MAPK-ERK pathway to affect its adhesion and infection
Abstract
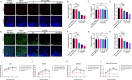
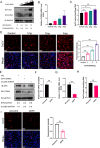
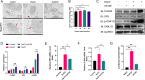
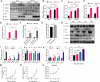
Duck circovirus (DuCV) is widely recognized as a prominent virus in China's duck farming industry, known for its ability to cause persistent infections and significant immunosuppression, which can lead to an increased susceptibility to secondary infections, posing a significant threat to the duck industry. Moreover, clinical evidence also indicates the potential vertical transmission of the virus through duck embryos to subsequent generations of ducklings. However, the limited availability of suitable cell lines for in vitro cultivation of DuCV has hindered further investigation into the molecular mechanisms underlying its infection and pathogenicity. In this study, we observed that oral DuCV infection in female breeding ducks can lead to oviduct, ovarian, and follicular infections. Subsequently, the infection can be transmitted to the fertilized eggs, resulting in the emergence of virus-carrying ducklings upon hatching. In contrast, the reproductive organs of male breeding ducks were unaffected by the virus, thus confirming that vertical transmission of DuCV primarily occurs through infection in female breeding ducks. By analyzing transcriptome sequencing data from the oviduct, we focused on claudin-2, a gene encoding the tight junction protein CLDN2 located on the cell membrane, which showed significantly increased expression in DuCV-infected oviducts of female breeding ducks. Notably, CLDN2 was confirmed to interact with the unique structural protein of DuCV, namely capsid protein (Cap), through a series of experimental approaches including co-immunoprecipitation (co-IP), GST pull-down, immunofluorescence, and adhesion-blocking assays. Furthermore, we demonstrated that the Cap protein binds to the extracellular loop structural domains EL1 and EL2 of CLDN2. Subsequently, by constructing a series of truncated bodies of the CLDN2 promoter region, we identified the transcription factor SP5 for CLDN2. Moreover, we found that DuCV infection triggers the activation of the MAPK-ERK signaling pathway in DEF cells and ducks, leading to an upregulation of SP5 and CLDN2 expression. This process ultimately leads to the transportation of mature CLDN2 to the cell surface, thereby facilitating increased virus adherence to the target organs. In conclusion, we discovered that DuCV utilizes host CLDN2 proteins to enhance adhesion and infection in oviducts and other target organs. Furthermore, we elucidated the signaling pathways involved in the interaction between DuCV Cap proteins and CLDN2, which provides valuable insights into the molecular mechanism underlying DuCV's infection and vertical transmission.
Importance: Although duck circovirus (DuCV) poses a widespread infection and a serious hazard to the duck industry, the molecular mechanisms underlying DuCV infection and transmission remain elusive. We initially demonstrated vertical transmission of DuCV through female breeding ducks by simulating natural infection. Furthermore, a differentially expressed membrane protein CLDN2 was identified on the DuCV-infected oviduct of female ducks, and its extracellular loop structural domains EL1 and EL2 were identified as the interaction sites of DuCV Cap proteins. Moreover, the binding of DuCV Cap to CLDN2 triggered the intracellular MAPK-ERK pathway and activated the downstream transcription factor SP5. Importantly, we demonstrated that intracellular Cap also interacts with SP5, leading to upregulation of CLDN2 transcription and facilitating enhanced adherence of DuCV to target tissue, thereby promoting viral infection and transmission. Our study sheds light on the molecular mechanisms underlying vertical transmission of DuCV, highlighting CLDN2 as a promising target for drug development against DuCV infection.
Keywords: MAPK-ERK pathway; adhesion; claudin-2; duck circovirus; vertical transmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Zhu F, Yin Z-T, Wang Z, Smith J, Zhang F, Martin F, Ogeh D, Hincke M, Lin F-B, Burt DW, Zhou Z-K, Hou S-S, Zhao Q-S, Li X-Q, Ding S-R, Li G-S, Yang F-X, Hao J-P, Zhang Z, Lu L-Z, Yang N, Hou Z-C. 2021. Three chromosome-level duck genome assemblies provide insights into genomic variation during domestication. Nat Commun 12:1–11. doi: 10.1038/s41467-021-26272-1 - DOI - PMC - PubMed
-
- FAO . 2021. Crops and livestock products. Available from: https://www.fao.org/faostat/en/#data.last access. Retrieved Nov 2021.
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

