Tim-3 Is Required for Regulatory T Cell-Mediated Promotion of T Cell Exhaustion and Viral Persistence during Chronic Lymphocytic Choriomeningitis Virus Infection
- PMID: 39345172
- PMCID: PMC11671103
- DOI: 10.4049/jimmunol.2400119
Tim-3 Is Required for Regulatory T Cell-Mediated Promotion of T Cell Exhaustion and Viral Persistence during Chronic Lymphocytic Choriomeningitis Virus Infection
Abstract
Expression of T cell Ig and mucin domain-containing protein 3 (Tim-3) is upregulated on regulatory T cells (Tregs) during chronic viral infections. In several murine and human chronic infections, the expression of Tim-3 is associated with poor control of viral burden and impaired antiviral immune responses. However, the role of Tim-3+ Tregs during persistent viral infections has not been fully defined. We employed an inducible Treg-specific Tim-3 loss-of-function (Tim-3 Treg knockout) murine model to dissect the role of Tim-3 on Tregs during chronic lymphocytic choriomeningitis virus infection. Tim-3 Treg knockout mice exhibited a decrease in morbidity, a more potent virus-specific T cell response, and a significant decrease in viral burden. These mice also had a reduction in the frequency of PD-1+Tim-3+ and PD-1+Tox+ gp33-specific exhausted CD8+ T cells. Our findings demonstrate that modulation of a single surface protein on Tregs can lead to a reduction in viral burden, limit T cell exhaustion, and enhance gp33-specific T cell response. These studies may help to identify Tim-3-directed therapies for the management of persistent infections and cancer.
Copyright © 2024 by The American Association of Immunologists, Inc.
Figures

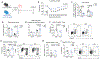
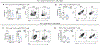




References
-
- Penaloza-MacMaster P, Kamphorst AO, Wieland A, Araki K, Iyer SS, West EE, O’Mara L, Yang S, Konieczny BT, Sharpe AH, Freeman GJ, Rudensky AY, and Ahmed R. 2014. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J Exp Med 211: 1905–1918. - PMC - PubMed
-
- Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, and Nelson DR. 2004. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology 40: 1062–1071. - PubMed
-
- Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, and Janssen HL. 2005. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 41: 771–778. - PubMed
-
- Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, and Chang KM. 2003. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology 38: 1437–1448. - PubMed
-
- Eggena MP, Barugahare B, Jones N, Okello M, Mutalya S, Kityo C, Mugyenyi P, and Cao H. 2005. Depletion of Regulatory T Cells in HIV Infection Is Associated with Immune Activation1. The Journal of Immunology 174: 4407–4414. - PubMed
MeSH terms
Substances
Grants and funding
- DP2 AI136598/AI/NIAID NIH HHS/United States
- S10OD032265/HHS | NIH | NIH Office of the Director (OD)
- R01 AI171483/AI/NIAID NIH HHS/United States
- R01CA206517/HHS | NIH | NCI | Center for Cancer Research (CCR)
- F31CA261039/HHS | NIH | NCI | Center for Cancer Research (CCR)
- R01 CA206517/CA/NCI NIH HHS/United States
- T32GM008208/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- T32CA082084/HHS | NIH | NCI | Center for Cancer Research (CCR)
- R01AI138504/HHS | NIH | NIAID | Division of Microbiology and Infectious Diseases (DMID)
- S10 OD032265/OD/NIH HHS/United States
- R01 AI138504/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

