Adjunctive intra-arterial tenecteplase after successful endovascular thrombectomy in patients with large vessel occlusion stroke (POST-TNK): Study rationale and design
- PMID: 39345180
- PMCID: PMC11556599
- DOI: 10.1177/23969873241286983
Adjunctive intra-arterial tenecteplase after successful endovascular thrombectomy in patients with large vessel occlusion stroke (POST-TNK): Study rationale and design
Abstract
Rationale: Adjunct intra-arterial alteplase has been shown to potentially improve clinical outcomes in patients with large vessel occlusion (LVO) stroke who have undergone successful endovascular thrombectomy. Tenecteplase, known for its enhanced fibrin specificity and extended activity duration, could potentially enhance outcomes in stroke patients after successful reperfusion when used as an adjunct intra-arterial therapy.
Aim: To explore the safety and efficacy of intra-arterial tenecteplase after successful endovascular thrombectomy in patients with LVO stroke.
Sample size: To randomize 498 participants 1:1 to receive intra-arterial tenecteplase or no intra-arterial adjunctive thrombolysis therapy.
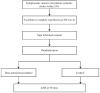
Methods and design: An investigator-initiated, prospective, randomized, open-label, blind-endpoint multicenter clinical trial. Eligible patients with anterior circulation LVO stroke presenting within 24 h from symptom onset (time last known well) and excellent to complete reperfusion (expanded Thrombolysis In Cerebral Infarction (eTICI) scale 2c-3) at endovascular thrombectomy are planned to be randomized.
Outcomes: The primary outcome is freedom from disability (modified Rankin Scale, mRS, of 0-1) at 90 days. The primary safety outcomes are mortality through 90 days and symptomatic intracranial hemorrhage within 48 h.
Discussion: The POST-TNK trial will evaluate the efficacy and safety of intra-arterial tenecteplase in patients with LVO stroke and excellent to complete reperfusion.
Keywords: Large vessel occlusion stroke; endovascular thrombectomy; intra-arterial tenecteplase.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JLS reports consulting fees for advising on rigorous and safe clinical trial design and conduct from Biogen, Boehringer Ingelheim, Genentech, Johnson&Johnson, Phenox, Phillips, Rapid Medical, and Roche. TNN discloses Associate Editor of Stroke, advisory board of Aruna Bio and, Brainomix. RGN reports consulting fees for advisory roles with Anaconda, Biogen, Cerenovus, Genentech, Philips, Hybernia, Hyperfine, Imperative Care, Medtronic, Phenox, Philips, Prolong Pharmaceuticals, Stryker Neurovascular, Shanghai Wallaby, Synchron, and stock options for advisory roles with Astrocyte, Brainomix, Cerebrotech, Ceretrieve, Corindus Vascular Robotics, CrestecBio Inc., Euphrates Vascular, Inc., Vesalio, Viz-AI, RapidPulse and Perfuze. RGN is one of the Principal Investigators of ENDOLOW trial. Funding for this project is provided by Cerenovus. RGN is the Principal Investigator of the DUSK trial. Funding for this project is provided by Stryker Neurovascular. RGN is an investor in Viz-AI, Perfuze, Cerebrotech, Reist/Q’Apel Medical, Truvic, Tulavi Therapeutics, Vastrax, Piraeus Medical, Brain4Care, Quantanosis AI, and Viseon. Other author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Intra-Arterial Tenecteplase Following Endovascular Reperfusion for Large Vessel Occlusion Acute Ischemic Stroke: The POST-TNK Randomized Clinical Trial.JAMA. 2025 Feb 18;333(7):579-588. doi: 10.1001/jama.2024.23466. JAMA. 2025. PMID: 39804681 Free PMC article. Clinical Trial.
-
Intra-arterial Alteplase Thrombolysis After Successful Thrombectomy for Acute Ischemic Stroke in the Posterior Circulation (IAT-TOP): Study protocol and rationale.Int J Stroke. 2025 Jul;20(6):750-755. doi: 10.1177/17474930251313940. Epub 2025 Jan 23. Int J Stroke. 2025. PMID: 39754489
-
Intra-arterial Tenecteplase for Acute Stroke After Successful Endovascular Therapy: The ANGEL-TNK Randomized Clinical Trial.JAMA. 2025 Jul 5:e2510800. doi: 10.1001/jama.2025.10800. Online ahead of print. JAMA. 2025. PMID: 40616323
-
Efficacy and safety of intra-arterial thrombolysis after endovascular reperfusion for acute ischemic stroke: a systematic review and meta-analysis of randomized trials.Int J Surg. 2025 Jun 1;111(6):4002-4008. doi: 10.1097/JS9.0000000000002404. Epub 2025 Apr 25. Int J Surg. 2025. PMID: 40277355 Free PMC article.
-
Intravenous thrombolysis with tenecteplase versus alteplase in acute ischemic stroke tandem occlusions: a systematic review and meta-analysis of current available literature.J Thromb Thrombolysis. 2025 Mar;58(3):411-419. doi: 10.1007/s11239-025-03084-4. Epub 2025 Mar 13. J Thromb Thrombolysis. 2025. PMID: 40082388
Cited by
-
Intra-Arterial Tenecteplase Following Endovascular Reperfusion for Large Vessel Occlusion Acute Ischemic Stroke: The POST-TNK Randomized Clinical Trial.JAMA. 2025 Feb 18;333(7):579-588. doi: 10.1001/jama.2024.23466. JAMA. 2025. PMID: 39804681 Free PMC article. Clinical Trial.
-
Intra-Arterial Tenecteplase After Successful Reperfusion in Large Vessel Occlusion Stroke: A Randomized Clinical Trial.JAMA Neurol. 2025 Jul 5:e252036. doi: 10.1001/jamaneurol.2025.2036. Online ahead of print. JAMA Neurol. 2025. PMID: 40616333 Free PMC article.
References
-
- Berkhemer O, Fransen P, Beumer D. A randomized trial of intraarterial treatment for acute ischemic stroke. New Engl J Med 2015; 372: 394–420. - PubMed
-
- Goyal M, Demchuk A, Menon B, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. New Engl J Med 2015; 372: 1019–1030. - PubMed
-
- Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenoust-pa vs. T-pa alone in stroke. New Engl J Med 2015; 372: 2285–2295. - PubMed
-
- Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. New Engl J Med 2015; 372: 2296–2306. - PubMed
-
- Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. New Engl J Med 2015; 372: 1009–1018. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical