Contribution of direct InhA inhibitors to novel drug regimens in a mouse model of tuberculosis
- PMID: 39345183
- PMCID: PMC11539229
- DOI: 10.1128/aac.00357-24
Contribution of direct InhA inhibitors to novel drug regimens in a mouse model of tuberculosis
Abstract
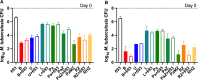
Isoniazid is an important first-line medicine to treat tuberculosis (TB). Isoniazid resistance increases the risk of poor treatment outcomes and development of multidrug resistance, and is driven primarily by mutations involving katG, encoding the prodrug-activating enzyme, rather than its validated target, InhA. The chemical tractability of InhA has fostered efforts to discover direct inhibitors of InhA (DIIs). In this study, we bridge the gap in understanding the potential contribution of DIIs to novel combination regimens and demonstrate a clear distinction of DIIs, like GSK693 and the newly described GSK138, from isoniazid, based on activity against clinical isolates and contribution to novel drug regimens. The results suggest that DIIs, specifically GSK138 and GSK693, could be promising partners in novel drug regimens, including those used against isoniazid-resistant TB, potentially enhancing their efficacy and/or preventing the selection of resistant mutants and supporting the continued exploration of InhA as a promising target for TB drug development.
Keywords: GSK138; GSK2505693A; GSK3081138A; GSK693; InhA inhibitor; mouse; tuberculosis; tuberculosis drugs.
Conflict of interest statement
L.E., J.R.-T., A.G.-P., R.G.D.R., and D.B.-A. are employees of, and shareholders in, GSK. A.M.-L. holds GSK stock and has patents issued during his employment at GSK. J.D.M. and V.S. are employees of GSK. The other authors declare no conflict of interest.
Figures





References
-
- World Health Organization . 2023. Global Tuberculosis Report 2023
-
- Dean AS, Zignol M, Cabibbe AM, Falzon D, Glaziou P, Cirillo DM, Köser CU, Gonzalez-Angulo LY, Tosas-Auget O, Ismail N, Tahseen S, Ama MCG, Skrahina A, Alikhanova N, Kamal SMM, Floyd K. 2020. Prevalence and genetic profiles of isoniazid resistance in tuberculosis patients: a multicountry analysis of cross-sectional data. PLoS Med 17:e1003008. doi:10.1371/journal.pmed.1003008 - DOI - PMC - PubMed
-
- Fregonese F, Ahuja SD, Akkerman OW, Arakaki-Sanchez D, Ayakaka I, Baghaei P, Bang D, Bastos M, Benedetti A, Bonnet M, et al. . 2018. Comparison of different treatments for isoniazid-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir Med 6:265–275. doi:10.1016/S2213-2600(18)30078-X - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

