Characterization and functional analysis of Toxoplasma Golgi-associated proteins identified by proximity labeling
- PMID: 39345210
- PMCID: PMC11559087
- DOI: 10.1128/mbio.02380-24
Characterization and functional analysis of Toxoplasma Golgi-associated proteins identified by proximity labeling
Abstract
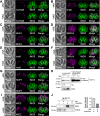
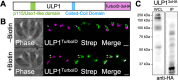
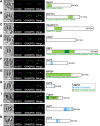
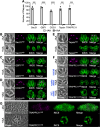
Toxoplasma gondii possesses a highly polarized secretory pathway that contains both broadly conserved eukaryotic organelles and unique apicomplexan organelles, which play essential roles in the parasite's lytic cycle. As in other eukaryotes, the T. gondii Golgi apparatus sorts and modifies proteins prior to their distribution to downstream organelles. Many of the typical trafficking factors found involved in these processes are missing from apicomplexan genomes, suggesting that these parasites have evolved unique proteins to fill these roles. Here, we identify a Golgi-localizing protein (ULP1), which is structurally similar to the eukaryotic trafficking factor p115/Uso1. We demonstrate that depletion of ULP1 leads to a dramatic reduction in parasite fitness that is the result of defects in microneme secretion, invasion, replication, and egress. Using ULP1 as bait for TurboID proximity labeling and immunoprecipitation, we identify 11 more Golgi-associated proteins and demonstrate that ULP1 interacts with the T. gondii-conserved oligomeric Golgi (COG) complex. These proteins include both conserved trafficking factors and parasite-specific proteins. Using a conditional knockdown approach, we assess the effect of each of these 11 proteins on parasite fitness. Together, this work reveals a diverse set of T. gondii Golgi-associated proteins that play distinct roles in the secretory pathway. As several of these proteins are absent outside of the Apicomplexa, they represent potential targets for the development of novel therapeutics against these parasites.
Importance: Apicomplexan parasites such as Toxoplasma gondii infect a large percentage of the world's population and cause substantial human disease. These widespread pathogens use specialized secretory organelles to infect their host cells, modulate host cell functions, and cause disease. While the functions of the secretory organelles are now better understood, the Golgi apparatus of the parasite remains largely unexplored, particularly regarding parasite-specific innovations that may help direct traffic intracellularly. In this work, we characterize ULP1, a protein that is unique to parasites but shares structural similarity to the eukaryotic trafficking factor p115/Uso1. We show that ULP1 plays an important role in parasite fitness and demonstrate that it interacts with the conserved oligomeric Golgi (COG) complex. We then use ULP1 proximity labeling to identify 11 additional Golgi-associated proteins, which we functionally analyze via conditional knockdown. This work expands our knowledge of the Toxoplasma Golgi apparatus and identifies potential targets for therapeutic intervention.
Keywords: ER exit sites; Golgi apparatus; Toxoplasma gondii; apicomplexan parasites; protein trafficking; secretory pathway; vesicular trafficking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Update of
-
Characterization and functional analysis of Toxoplasma Golgi-associated proteins identified by proximity labelling.bioRxiv [Preprint]. 2024 May 22:2024.02.02.578703. doi: 10.1101/2024.02.02.578703. bioRxiv. 2024. Update in: mBio. 2024 Nov 13;15(11):e0238024. doi: 10.1128/mbio.02380-24. PMID: 38352341 Free PMC article. Updated. Preprint.
References
-
- Sow SO, Muhsen K, Nasrin D, Blackwelder WC, Wu Y, Farag TH, Panchalingam S, Sur D, Zaidi AKM, Faruque ASG, et al. . 2016. The burden of Cryptosporidium diarrheal disease among children < 24 months of age in moderate/high mortality regions of sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis 10:e0004729. doi:10.1371/journal.pntd.0004729 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- Milton Gottlieb Endowment Award/UC | University of California, Los Angeles (UCLA)
- T32 AI007323/AI/NIAID NIH HHS/United States
- Beckman Scholars Award/Arnold and Mabel Beckman Foundation (AMBF)
- GM089778/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- T32 GM007185/GM/NIGMS NIH HHS/United States
- AI123360/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R35 GM153408/GM/NIGMS NIH HHS/United States
- GM007185/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- AI007323/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 GM089778/GM/NIGMS NIH HHS/United States
- Whitcome Fellowship/UC | University of California, Los Angeles (UCLA)
- R01 AI123360/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

