This is a preprint.
Spinal neuron diversity scales exponentially with swim-to-limb transformation during frog metamorphosis
- PMID: 39345366
- PMCID: PMC11430061
- DOI: 10.1101/2024.09.20.614050
Spinal neuron diversity scales exponentially with swim-to-limb transformation during frog metamorphosis
Abstract
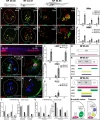
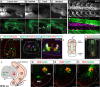
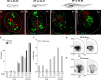
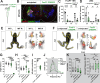
Vertebrates exhibit a wide range of motor behaviors, ranging from swimming to complex limb-based movements. Here we take advantage of frog metamorphosis, which captures a swim-to-limb-based movement transformation during the development of a single organism, to explore changes in the underlying spinal circuits. We find that the tadpole spinal cord contains small and largely homogeneous populations of motor neurons (MNs) and V1 interneurons (V1s) at early escape swimming stages. These neuronal populations only modestly increase in number and subtype heterogeneity with the emergence of free swimming. In contrast, during frog metamorphosis and the emergence of limb movement, there is a dramatic expansion of MN and V1 interneuron number and transcriptional heterogeneity, culminating in cohorts of neurons that exhibit striking molecular similarity to mammalian motor circuits. CRISPR/Cas9-mediated gene disruption of the limb MN and V1 determinants FoxP1 and Engrailed-1, respectively, results in severe but selective deficits in tail and limb function. Our work thus demonstrates that neural diversity scales exponentially with increasing behavioral complexity and illustrates striking evolutionary conservation in the molecular organization and function of motor circuits across species.
Keywords: conservation; diversity; interneuron; locomotion; motor neurons.
Conflict of interest statement
DECLARATION OF INTERESTS We declare no competing interests.
Figures




References
-
- Biewener A., and Patek S. (2018). Animal Locomotion (Oxford University Press; ).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
