This is a preprint.
Zika virus NS3 drives the assembly of a viroplasm-like structure
- PMID: 39345390
- PMCID: PMC11429906
- DOI: 10.1101/2024.09.16.613201
Zika virus NS3 drives the assembly of a viroplasm-like structure
Abstract
Zika virus (ZIKV) is a mosquito-transmitted flavivirus that caused an epidemic in 2015-2016 in the Americas and raised serious global health concerns due to its association with congenital brain developmental defects in infected pregnancies. Upon infection, ZIKV assembles virus particles in a virus-generated toroidal compartment next to the nucleus called the replication factory, or viroplasm, which forms by remodeling the host cell endoplasmic reticulum (ER). How the viral proteins control viroplasm assembly remains unknown. Here we show that the ZIKV non-structural protein 3 (NS3) is sufficient to drive the assembly of a viroplasm-like structure (VLS) in human cells. NS3 encodes a dual-function protease and RNA helicase. The VLS is similar to the ZIKV viroplasm in its assembly near centrosomes at the nuclear periphery, its deformation of the nuclear membrane, its recruitment of ER, Golgi, and dsRNA, and its association with microtubules at its surface. While sufficient to generate a VLS, NS3 is less efficient in several aspects compared to viroplasm formation upon ZIKV infection. We further show that the helicase domain and not the protease domain is required for optimal VLS assembly and dsRNA recruitment. Overall, this work advances our understanding of the mechanism of viroplasm assembly by ZIKV and likely will extend to other flaviviruses.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest.
Figures
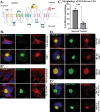
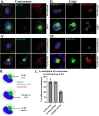
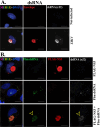
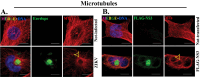
Similar articles
-
Coordination of Zika Virus Infection and Viroplasm Organization by Microtubules and Microtubule-Organizing Centers.Cells. 2021 Nov 27;10(12):3335. doi: 10.3390/cells10123335. Cells. 2021. PMID: 34943843 Free PMC article.
-
Zika Virus NS2A-Mediated Virion Assembly.mBio. 2019 Oct 29;10(5):e02375-19. doi: 10.1128/mBio.02375-19. mBio. 2019. PMID: 31662457 Free PMC article.
-
Nucleo-Cytoplasmic Transport of ZIKV Non-Structural 3 Protein Is Mediated by Importin-α/β and Exportin CRM-1.J Virol. 2023 Jan 31;97(1):e0177322. doi: 10.1128/jvi.01773-22. Epub 2022 Dec 8. J Virol. 2023. PMID: 36475764 Free PMC article.
-
Zika virus structural biology and progress in vaccine development.Biotechnol Adv. 2018 Jan-Feb;36(1):47-53. doi: 10.1016/j.biotechadv.2017.09.004. Epub 2017 Sep 12. Biotechnol Adv. 2018. PMID: 28916391 Review.
-
Immune Evasion Strategies Used by Zika Virus to Infect the Fetal Eye and Brain.Viral Immunol. 2020 Jan/Feb;33(1):22-37. doi: 10.1089/vim.2019.0082. Epub 2019 Nov 5. Viral Immunol. 2020. PMID: 31687902 Free PMC article. Review.
References
-
- Bhatnagar J, Rabeneck DB, Martines RB, Reagan-Steiner S, Ermias Y, Estetter LB, Suzuki T, Ritter J, Keating MK, Hale G, Gary J, Muehlenbachs A, Lambert A, Lanciotti R, Oduyebo T, Meaney-Delman D, Bolanos F, Saad EA, Shieh WJ, Zaki SR. 2017. Zika Virus RNA Replication and Persistence in Brain and Placental Tissue. Emerg Infect Dis 23:405–414. - PMC - PubMed
-
- Ong CW. 2016. Zika virus: an emerging infectious threat. Intern Med J 46:525–30. - PubMed
-
- Musso D, Ko AI, Baud D. 2019. Zika Virus Infection — After the Pandemic. New England Journal of Medicine 381:1444–1457. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
