This is a preprint.
Spatial microenvironments tune immune response dynamics in the Drosophila larval fat body
- PMID: 39345471
- PMCID: PMC11429692
- DOI: 10.1101/2024.09.12.612587
Spatial microenvironments tune immune response dynamics in the Drosophila larval fat body
Abstract
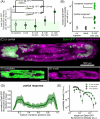
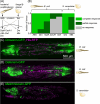
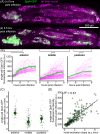
Immune responses in tissues display intricate patterns of gene expression that vary across space and time. While such patterns have been increasingly linked to disease outcomes, the mechanisms that generate them and the logic behind them remain poorly understood. As a tractable model of spatial immune responses, we investigated heterogeneous expression of antimicrobial peptides in the larval fly fat body, an organ functionally analogous to the liver. To capture the dynamics of immune response across the full tissue at single-cell resolution, we established live light sheet fluorescence microscopy of whole larvae. We discovered that expression of antimicrobial peptides occurs in a reproducible spatial pattern, with enhanced expression in the anterior and posterior lobes of the fat body. This pattern correlates with microbial localization via blood flow but is not caused by it: loss of heartbeat suppresses microbial transport but leaves the expression pattern unchanged. This result suggests that regions of the tissue most likely to encounter microbes via blood flow are primed to produce antimicrobials. Spatial transcriptomics revealed that these immune microenvironments are defined by genes spanning multiple biological processes, including lipid-binding proteins that regulate host cell death by the immune system. In sum, the larval fly fat body exhibits spatial compartmentalization of immune activity that resembles the strategic positioning of immune cells in mammals, such as in the liver, gut, and lymph nodes. This finding suggests that tissues may share a conserved spatial organization that optimizes immune responses for antimicrobial efficacy while preventing excessive self-damage.
Figures


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
T-bet expressing Tr1 cells driven by dietary signals dominate the small intestinal immune landscape.bioRxiv [Preprint]. 2025 Jul 4:2025.06.30.662190. doi: 10.1101/2025.06.30.662190. bioRxiv. 2025. PMID: 40747421 Free PMC article. Preprint.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults.Cochrane Database Syst Rev. 2016 Apr 21;4(4):CD009016. doi: 10.1002/14651858.CD009016.pub2. Cochrane Database Syst Rev. 2016. PMID: 27098439 Free PMC article.
References
-
- Kastenmüller W., Torabi-Parizi P., Subramanian N., Lämmermann T. & Germain R. N. A Spatially-Organized Multicellular Innate Immune Response in Lymph Nodes Limits Systemic Pathogen Spread. Cell 150. Publisher: Elsevier, 1235–1248. issn: 0092–8674. 10.1016/j.cell.2012.07.021 (2024) (Sept. 2012). - DOI - PMC - PubMed
-
- Williams C. G. et al. Spatial transcriptomics maps molecular and cellular requirements for CD4+ T cell-dependent immunity to malaria. bioRxiv. Publisher: Cold Spring Harbor Laboratory _eprint: https://www.biorxiv.org/content/early/2023/02/23/2023.02.23.529309.full.pdf https://www.biorxiv.org/content/early/2023/02/23/2023.02.23.529309 (2023).
-
- Centofanti E. et al. The spread of interferon-γ in melanomas is highly spatially confined, driving nongenetic variability in tumor cells. Proceedings of the National Academy of Sciences 120. Publisher: Proceedings of the National Academy of Sciences, e2304190120. 10.1073/pnas.2304190120 (2024) (Aug. 2023). - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
