This is a preprint.
Unique Transcriptomic Cell Types of the Granular Retrosplenial Cortex are Preserved Across Mice and Rats Despite Dramatic Changes in Key Marker Genes
- PMID: 39345493
- PMCID: PMC11429737
- DOI: 10.1101/2024.09.17.613545
Unique Transcriptomic Cell Types of the Granular Retrosplenial Cortex are Preserved Across Mice and Rats Despite Dramatic Changes in Key Marker Genes
Abstract
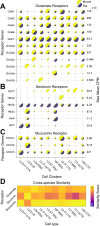
The granular retrosplenial cortex (RSG) supports key functions ranging from memory consolidation to spatial navigation. The mouse RSG contains several cell types that are remarkably distinct from those found in other cortical regions. This includes the physiologically and transcriptomically unique low rheobase neuron that is the dominant cell-type in RSG layers 2/3 (L2/3 LR), as well as the similarly exclusive pyramidal cells that comprise much of RSG layer 5a (L5a RSG). While the functions of the RSG are extensively studied in both mice and rats, it remains unknown if the transcriptomically unique cell types of the mouse RSG are evolutionarily conserved in rats. Here, we show that mouse and rat RSG not only contain the same cell types, but key subtypes including the L2/3 LR and L5a RSG neurons are amplified in their representations in rats compared to mice. This preservation of cell types in male and female rats happens despite dramatic changes in key cell-type-specific marker genes, with the Scnn1a expression that selectively tags mouse L5a RSG neurons completely absent in rats. Important for Cre-driver line development, we identify alternative, cross-species genes that can be used to selectively target the cell types of the RSG in both mice and rats. Our results show that the unique cell types of the RSG are evolutionarily conserved across millions of years of evolution between mice and rats, but also emphasize stark species-specific differences in marker genes that need to be considered when making cell-type-specific transgenic lines of mice versus rats.
Figures





Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
A cross-species analysis of neuroanatomical covariance sex differences in humans and mice.Biol Sex Differ. 2025 Jul 1;16(1):47. doi: 10.1186/s13293-025-00728-1. Biol Sex Differ. 2025. PMID: 40598550 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
