This is a preprint.
A chronic murine model of pulmonary Acinetobacter baumannii infection enabling the investigation of late virulence factors, long-term antibiotic treatments, and polymicrobial infections
- PMID: 39345519
- PMCID: PMC11429896
- DOI: 10.1101/2024.09.17.613469
A chronic murine model of pulmonary Acinetobacter baumannii infection enabling the investigation of late virulence factors, long-term antibiotic treatments, and polymicrobial infections
Update in
-
A chronic Acinetobacter baumannii pneumonia model to study long-term virulence factors, antibiotic treatments, and polymicrobial infections.Nat Commun. 2025 Aug 15;16(1):7617. doi: 10.1038/s41467-025-62655-4. Nat Commun. 2025. PMID: 40817088 Free PMC article.
Abstract
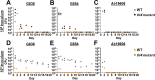
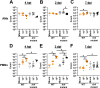
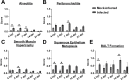
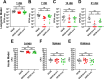
Acinetobacter baumannii can cause prolonged infections that disproportionately affect immunocompromised populations. Our understanding of A. baumannii respiratory pathogenesis relies on an acute murine infection model with limited clinical relevance that employs an unnaturally high number of bacteria and requires the assessment of bacterial load at 24-36 hours post-infection. Here, we demonstrate that low intranasal inoculums in immunocompromised mice with a tlr4 mutation leads to reduced inflammation, allowing for persistent infections lasting at least 3 weeks. Using this "chronic infection model," we determined the adhesin InvL is an imperative virulence factor required during later stages of infection, despite being dispensable in the early phase. We also demonstrate that the chronic model enables the distinction between antibiotics that, although initially reduce bacterial burden, either lead to complete clearance or result in the formation of bacterial persisters. To illustrate how our model can be applied to study polymicrobial infections, we inoculated mice with an active A. baumannii infection with Staphylococcus aureus or Klebsiella pneumoniae. We found that S. aureus exacerbates the infection, while K. pneumoniae enhances A. baumannii clearance. In all, the chronic model overcomes some limitations of the acute pulmonary model, expanding our capabilities to study of A. baumannii pathogenesis and lays the groundwork for the development of similar models for other important opportunistic pathogens.
Keywords: Acinetobacter baumannii; Antibiotic treatments; Murine model; Pathogenesis; Polymicrobial infections; Virulence factors.
Figures


References
-
- Cerqueira GM, Peleg AY. 2011. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life 63:1055–1060. - PubMed
-
- Di Venanzio G, Flores-Mireles AL, Calix JJ, Haurat MF, Scott NE, Palmer LD, Potter RF, Hibbing ME, Friedman L, Wang B, Dantas G, Skaar EP, Hultgren SJ, Feldman MF. 2019. Urinary tract colonization is enhanced by a plasmid that regulates uropathogenic Acinetobacter baumannii chromosomal genes. Nat Commun 10. - PMC - PubMed
-
- Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, Han C, Bisignano C, Rao P, Wool E, Johnson SC, Browne AJ, Chipeta MG, Fell F, Hackett S, Haines-Woodhouse G, Kashef Hamadani BH, Kumaran EAP, McManigal B, Agarwal R, Akech S, Albertson S, Amuasi J, Andrews J, Aravkin A, Ashley E, Bailey F, Baker S, Basnyat B, Bekker A, Bender R, Bethou A, Bielicki J, Boonkasidecha S, Bukosia J, Carvalheiro C, Castañeda-Orjuela C, Chansamouth V, Chaurasia S, Chiurchiù S, Chowdhury F, Cook AJ, Cooper B, Cressey TR, Criollo-Mora E, Cunningham M, Darboe S, Day NPJ, De Luca M, Dokova K, Dramowski A, Dunachie SJ, Eckmanns T, Eibach D, Emami A, Feasey N, Fisher-Pearson N, Forrest K, Garrett D, Gastmeier P, Giref AZ, Greer RC, Gupta V, Haller S, Haselbeck A, Hay SI, Holm M, Hopkins S, Iregbu KC, Jacobs J, Jarovsky D, Javanmardi F, Khorana M, Kissoon N, Kobeissi E, Kostyanev T, Krapp F, Krumkamp R, Kumar A, Kyu HH, Lim C, Limmathurotsakul D, Loftus MJ, Lunn M, Ma J, Mturi N, Munera-Huertas T, Musicha P, Mussi-Pinhata MM, Nakamura T, Nanavati R, Nangia S, Newton P, Ngoun C, Novotney A, Nwakanma D, Obiero CW, Olivas-Martinez A, Olliaro P, Ooko E, Ortiz-Brizuela E, Peleg AY, Perrone C, Plakkal N, Ponce-de-Leon A, Raad M, Ramdin T, Riddell A, Roberts T, Robotham JV, Roca A, Rudd KE, Russell N, Schnall J, Scott JAG, Shivamallappa M, Sifuentes-Osornio J, Steenkeste N, Stewardson AJ, Stoeva T, Tasak N, Thaiprakong A, Thwaites G, Turner C, Turner P, van Doorn HR, Velaphi S, Vongpradith A, Vu H, Walsh T, Waner S, Wangrangsimakul T, Wozniak T, Zheng P, Sartorius B, Lopez AD, Stergachis A, Moore C, Dolecek C, Naghavi M. 2022. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet 399:629–655. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
