This is a preprint.
Comparison of Explainable AI Models for MRI-based Alzheimer's Disease Classification
- PMID: 39345522
- PMCID: PMC11429733
- DOI: 10.1101/2024.09.17.613560
Comparison of Explainable AI Models for MRI-based Alzheimer's Disease Classification
Abstract
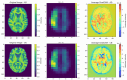
Deep learning models based on convolutional neural networks (CNNs) have been used to classify Alzheimer's disease or infer dementia severity from 3D T1-weighted brain MRI scans. Here, we examine the value of adding occlusion sensitivity analysis (OSA) and gradient-weighted class activation mapping (Grad-CAM) to these models to make the results more interpretable. Much research in this area focuses on specific datasets such as the Alzheimer's Disease Neuroimaging Initiative (ADNI) or National Alzheimer's Coordinating Center (NACC), which assess people of North American, predominantly European ancestry, so we examine how well models trained on these data generalize to a new population dataset from India (NIMHANS cohort). We also evaluate the benefit of using a combined dataset to train the CNN models. Our experiments show feature localization consistent with knowledge of AD from other methods. OSA and Grad-CAM resolve features at different scales to help interpret diagnostic inferences made by CNNs.
Keywords: Alzheimer’s Disease; Deep Learning; Grad-CAM; Magnetic Resonance Imaging; Occlusion Sensitivity Analysis.
Figures


References
-
- New AI legislation’s reach extends into European Healthcare. Osborne Clarke. (2024). https://www.osborneclarke.com/insights/new-ai-legislations-reach-extends....
-
- Selvaraju R. R., et al. (2017). Grad-CAM: Visual explanations from deep networks via gradient-based localization. In Proc. IEEE International Conference on Computer Vision (pp. 618–626).
-
- Zhang Y., et al. (2021). Grad-CAM helps interpret the deep learning models trained to classify multiple sclerosis types using clinical brain magnetic resonance imaging. NeuroImage: Clinical, 30, 102642. - PubMed
-
- Qin C., et al. (2021). A large-scale multimodal neuroimaging dataset to identify brain disorders based on anatomical and functional markers. Scientific Data, 8(1), 1–21.
-
- Sarraf S., & Tofighi G. (2022). Classification of Alzheimer’s disease using 3D convolutional neural networks and explainable artificial intelligence. Informatics in Medicine Unlocked, 26, 100732.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
