This is a preprint.
Integrating collecting systems in kidney organoids through fusion of distal nephron to ureteric bud
- PMID: 39345524
- PMCID: PMC11429897
- DOI: 10.1101/2024.09.19.613645
Integrating collecting systems in kidney organoids through fusion of distal nephron to ureteric bud
Update in
-
Integrating collecting systems in human kidney organoids through fusion of distal nephron to ureteric bud.Cell Stem Cell. 2025 Jul 3;32(7):1055-1070.e8. doi: 10.1016/j.stem.2025.04.008. Epub 2025 May 8. Cell Stem Cell. 2025. PMID: 40345193
Abstract
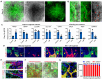
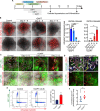
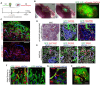
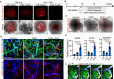
The kidney maintains homeostasis through an array of parallel nephrons, which all originate in development as isolated epithelial structures that later fuse through their distal poles to a system of collecting ducts (CD). This connection is required to generate functional nephrons by providing a pathway for excretion of metabolic waste and byproducts. Currently, methods for differentiating human pluripotent stem cells into kidney organoids generate nephrons that lack CDs and instead terminate as blind-ended tubules. Here we describe a developmentally inspired system that addresses this deficiency through assembly of induced nephrogenic mesenchyme with ureteric bud (UB) tissues, the embryonic building blocks of the kidney's collecting system. The UB progenitors grow and develop into a network of CDs within the organoid, and importantly, they functionally integrate with the nephrons through recapitulating fusion between the distal tubule and CD to create a continuous epithelial lumen. We further showed that proximal-distal nephron specification, fusion frequency, and maturation of the CD can be augmented through temporal manipulation of developmental signaling pathways. This work provides a platform for interrogating the principles and mechanisms underlying nephron-UB fusion and a framework for engineering unobstructed nephrons with patterned collecting systems, an important step toward the de novo generation of functional kidney tissue.
Conflict of interest statement
Declaration of Interests M.S., K.W.M., and J.V.B. are co-inventors on pending UB organoid patents, and M.S. and K.W.M. are coinventors on pending integrated organoid technologies described herein. The other authors have no competing financial interests to declare.
Figures



References
-
- Mugford J.W., Sipila P., McMahon J.A., and McMahon A.P. (2008). Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol 324, 88–98. 10.1016/j.ydbio.2008.09.010. - DOI - PMC - PubMed
-
- Taguchi A., Kaku Y., Ohmori T., Sharmin S., Ogawa M., Sasaki H., and Nishinakamura R. (2014). Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67. 10.1016/j.stem.2013.11.010. - DOI - PubMed
-
- Georgas K., Rumballe B., Valerius M.T., Chiu H.S., Thiagarajan R.D., Lesieur E., Aronow B.J., Brunskill E.W., Combes A.N., Tang D., et al. (2009). Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol 332, 273–286. 10.1016/j.ydbio.2009.05.578. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
