This is a preprint.
RalB uncoupled exocyst mediates endothelial Weibel-Palade body exocytosis
- PMID: 39345530
- PMCID: PMC11429928
- DOI: 10.1101/2024.09.16.613344
RalB uncoupled exocyst mediates endothelial Weibel-Palade body exocytosis
Update in
-
RalB uncoupling from exocyst is required for endothelial Weibel-Palade body exocytosis.Mol Biol Cell. 2025 May 1;36(5):ar62. doi: 10.1091/mbc.E24-11-0493. Epub 2025 Apr 2. Mol Biol Cell. 2025. PMID: 40172988
Abstract
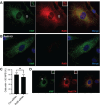
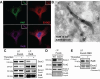
Ras-like (Ral) GTPases play essential regulatory roles in many cellular processes, including exocytosis. Cycling between GDP- and GTP-bound states, Ral GTPases function as molecular switches and regulate effectors, specifically the multi-subunit tethering complex exocyst. Here, we show that Ral isoform RalB controls regulated exocytosis of Weibel-Palade bodies (WPBs), the specialized endothelial secretory granules that store hemostatic protein von Willebrand factor. Remarkably, unlike typical small GTPase-effector interactions, RalB binds exocyst in its GDP-bound state in resting endothelium. Upon endothelial cell stimulation, exocyst is uncoupled from RalB-GTP resulting in WPB tethering and exocytosis. Furthermore, we report that PKC-dependent phosphorylation of the C-terminal hypervariable region (HVR) of RalB modulates its dynamic interaction with exocyst in endothelium. Exocyst preferentially interacts with phosphorylated RalB in resting endothelium. Dephosphorylation of RalB either by endothelial cell stimulation, or PKC inhibition, or expression of nonphosphorylatable mutant at a specific serine residue of RalB HVR, disengages exocyst and augments WPB exocytosis, resembling RalB exocyst-binding site mutant. In summary, it is the uncoupling of exocyst from RalB that mediates endothelial Weibel-Palade body exocytosis. Our data shows that Ral function may be more dynamically regulated by phosphorylation and may confer distinct functionality given high degree of homology and the shared set of effector protein between the two Ral isoforms.
Conflict of interest statement
Conflict of Interest The authors declare no financial conflict of interest.
Figures





Similar articles
-
RalB uncoupling from exocyst is required for endothelial Weibel-Palade body exocytosis.Mol Biol Cell. 2025 May 1;36(5):ar62. doi: 10.1091/mbc.E24-11-0493. Epub 2025 Apr 2. Mol Biol Cell. 2025. PMID: 40172988
-
Small GTP-binding protein Ral modulates regulated exocytosis of von Willebrand factor by endothelial cells.Arterioscler Thromb Vasc Biol. 2001 Jun;21(6):899-904. doi: 10.1161/01.atv.21.6.899. Arterioscler Thromb Vasc Biol. 2001. PMID: 11397694
-
VWF maturation and release are controlled by 2 regulators of Weibel-Palade body biogenesis: exocyst and BLOC-2.Blood. 2020 Dec 10;136(24):2824-2837. doi: 10.1182/blood.2020005300. Blood. 2020. PMID: 32614949 Free PMC article.
-
A new look at an old body: molecular determinants of Weibel-Palade body composition and von Willebrand factor exocytosis.J Thromb Haemost. 2024 May;22(5):1290-1303. doi: 10.1016/j.jtha.2024.01.015. Epub 2024 Feb 1. J Thromb Haemost. 2024. PMID: 38307391 Review.
-
Lifecycle of Weibel-Palade bodies.Hamostaseologie. 2017 Jan 31;37(1):13-24. doi: 10.5482/HAMO-16-07-0021. Epub 2016 Dec 22. Hamostaseologie. 2017. PMID: 28004844 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
