This is a preprint.
Abstinence from cocaine self-administration promotes microglia pruning of astrocytes which drives cocaine-seeking behavior
- PMID: 39345569
- PMCID: PMC11429948
- DOI: 10.1101/2024.09.20.614128
Abstinence from cocaine self-administration promotes microglia pruning of astrocytes which drives cocaine-seeking behavior
Update in
-
Abstinence from cocaine self-administration promotes microglial pruning of astrocytes, which drives cocaine-seeking behavior.Cell Rep. 2025 Aug 26;44(8):116137. doi: 10.1016/j.celrep.2025.116137. Epub 2025 Aug 12. Cell Rep. 2025. PMID: 40802516
Abstract
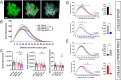
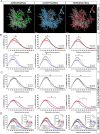
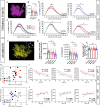
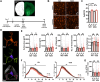
Rodent drug self-administration leads to compromised ability of astrocytes to maintain glutamate homeostasis within the brain's reward circuitry, as well as reductions in surface area, volume, and synaptic colocalization of astrocyte membranes. However, the mechanisms driving astrocyte responses to cocaine are unknown. Here, we report that long-access cocaine self-administration followed by prolonged home cage abstinence results in decreased branching complexity of nucleus accumbens astrocytes, characterized by the loss of peripheral processes. Using a combination of confocal fluorescence microcopy and immuno-gold electron microscopy, we show that alterations in astrocyte structural features are driven by microglia phagocytosis, as labeled astrocyte membranes are found within microglia phagolysosomes. Inhibition of complement C3-mediated phagocytosis using the neutrophil inhibitory peptide (NIF) rescued astrocyte structure and decreased cocaine seeking behavior following cocaine self-administration and abstinence. Collectively, these results provide evidence for microglia pruning of accumbens astrocytes across cocaine abstinence which mediates cocaine craving.
Keywords: astrocytes; cocaine; drug seeking; microglia; nucleus accumbens; phagocytosis; relapse.
Conflict of interest statement
Declaration of interests: The authors declare no competing interests.
Figures


References
-
- Shigetomi E., Bushong E.A., Haustein M.D., Tong X., Jackson-Weaver O., Kracun S., Xu J., Sofroniew M.V., Ellisman M.H., and Khakh B.S. (2013). Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol 141, 633–647. 10.1085/jgp.201210949. - DOI - PMC - PubMed
-
- Chai H., Diaz-Castro B., Shigetomi E., Monte E., Octeau J.C., Yu X., Cohn W., Rajendran P.S., Vondriska T.M., Whitelegge J.P., et al. (2017). Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 95, 531–549 e539. 10.1016/j.neuron.2017.06.029. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
