This is a preprint.
EFFECTS OF SPINAL TRANSECTION AND LOCOMOTOR SPEED ON MUSCLE SYNERGIES OF THE CAT HINDLIMB
- PMID: 39345603
- PMCID: PMC11429932
- DOI: 10.1101/2024.09.19.613891
EFFECTS OF SPINAL TRANSECTION AND LOCOMOTOR SPEED ON MUSCLE SYNERGIES OF THE CAT HINDLIMB
Update in
-
Effects of spinal transection and locomotor speed on muscle synergies of the cat hindlimb.J Physiol. 2025 May;603(10):3061-3088. doi: 10.1113/JP288089. Epub 2025 May 5. J Physiol. 2025. PMID: 40321018 Free PMC article.
Abstract
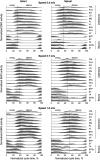
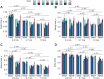
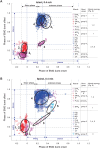
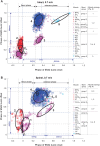
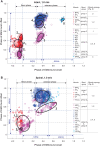
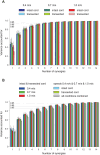
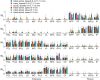
It was suggested that during locomotion, the nervous system controls movement by activating groups of muscles, or muscle synergies. Analysis of muscle synergies can reveal the organization of spinal locomotor networks and how it depends on the state of the nervous system, such as before and after spinal cord injury, and on different locomotor conditions, including a change in speed. The goal of this study was to investigate the effects of spinal transection and locomotor speed on hindlimb muscle synergies and their time-dependent activity patterns in adult cats. EMG activities of 15 hindlimb muscles were recorded in 9 adult cats of either sex during tied-belt treadmill locomotion at speeds of 0.4, 0.7, and 1.0 m/s before and after recovery from a low thoracic spinal transection. We determined EMG burst groups using cluster analysis of EMG burst onset and offset times and muscle synergies using non-negative matrix factorization. We found five major EMG burst groups and five muscle synergies in each of six experimental conditions (2 states × 3 speeds). In each case, the synergies accounted for at least 90% of muscle EMG variance. Both spinal transection and locomotion speed modified subgroups of EMG burst groups and the composition and activation patterns of selected synergies. However, these changes did not modify the general organization of muscle synergies. Based on the obtained results, we propose an organization for a pattern formation network of a two-level central pattern generator that can be tested in neuromechanical simulations of spinal circuits controlling cat locomotion.
Keywords: Locomotion; central pattern generator; muscle synergies; spinal transection.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures





Similar articles
-
Effects of spinal transection and locomotor speed on muscle synergies of the cat hindlimb.J Physiol. 2025 May;603(10):3061-3088. doi: 10.1113/JP288089. Epub 2025 May 5. J Physiol. 2025. PMID: 40321018 Free PMC article.
-
Spinal control of muscle synergies for adult mammalian locomotion.J Physiol. 2019 Jan;597(1):333-350. doi: 10.1113/JP277018. Epub 2018 Nov 10. J Physiol. 2019. PMID: 30334575 Free PMC article.
-
The Spinal Control of Backward Locomotion.J Neurosci. 2021 Jan 27;41(4):630-647. doi: 10.1523/JNEUROSCI.0816-20.2020. Epub 2020 Nov 25. J Neurosci. 2021. PMID: 33239399 Free PMC article.
-
The "beneficial" effects of locomotor training after various types of spinal lesions in cats and rats.Prog Brain Res. 2015;218:173-98. doi: 10.1016/bs.pbr.2014.12.009. Epub 2015 Mar 29. Prog Brain Res. 2015. PMID: 25890137 Review.
-
Muscle synergies during locomotion in the cat: a model for motor cortex control.J Physiol. 2008 Mar 1;586(5):1239-45. doi: 10.1113/jphysiol.2007.146605. Epub 2008 Jan 17. J Physiol. 2008. PMID: 18202098 Free PMC article. Review.
References
-
- Berkowitz A (2019) Expanding our horizons: central pattern generation in the context of complex activity sequences. J Exp Biol 222. - PubMed
-
- Bernstein NA (1967) The Coordination and Regulation of Movements. New York: Pergamon Press.
-
- Bernstein NA (2021) On the Construction of Movements. 1947. In: Bernstein’s Construction of Movements: The Original Text and Commentaries. 2020 (Latash ML, ed). New York, London: Routledge.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
