This is a preprint.
Distinct H3K9me3 heterochromatin maintenance dynamics govern different gene programs and repeats in pluripotent cells
- PMID: 39345615
- PMCID: PMC11429881
- DOI: 10.1101/2024.09.16.613328
Distinct H3K9me3 heterochromatin maintenance dynamics govern different gene programs and repeats in pluripotent cells
Update in
-
Distinct H3K9me3 heterochromatin maintenance dynamics govern different gene programmes and repeats in pluripotent cells.Nat Cell Biol. 2024 Dec;26(12):2115-2128. doi: 10.1038/s41556-024-01547-z. Epub 2024 Oct 31. Nat Cell Biol. 2024. PMID: 39482359
Abstract
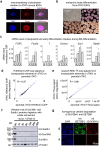
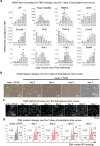
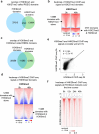
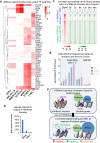
H3K9me3-heterochromatin, established by lysine methyltransferases (KMTs) and compacted by HP1 isoforms, represses alternative lineage genes and DNA repeats. Our understanding of H3K9me3-heterochromatin stability is presently limited to individual domains and DNA repeats. We engineered Suv39h2 KO mouse embryonic stem cells to degrade remaining two H3K9me3-KMTs within one hour and found that both passive dilution and active removal contribute to H3K9me3 decay within 12-24 hours. We discovered four different H3K9me3 decay rates across the genome and chromatin features and transcription factor binding patterns that predict the stability classes. A "binary switch" governs heterochromatin compaction, with HP1 rapidly dissociating from heterochromatin upon KMTs' depletion and a particular threshold level of HP1 limiting pioneer factor binding, chromatin opening, and exit from pluripotency within 12 hr. Unexpectedly, receding H3K9me3 domains unearth residual HP1β peaks enriched with heterochromatin-inducing proteins. Our findings reveal distinct H3K9me3-heterochromatin maintenance dynamics governing gene networks and repeats that together safeguard pluripotency.
Conflict of interest statement
Competing Interests Statement: The authors declare no competing interests.
Figures













References
-
- Matsui T. et al. Proviral silencing in embryonic stem cells requires the histone methyltransferase ESET. Nature 464, 927–931 (2010). - PubMed
-
- Tsumura A. et al. Maintenance of self-renewal ability of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a and Dnmt3b. Genes Cells 11, 805–814 (2006). - PubMed
Methods-only references
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
