This is a preprint.
Chromosomal instability increases radiation sensitivity
- PMID: 39345631
- PMCID: PMC11429890
- DOI: 10.1101/2024.09.13.612942
Chromosomal instability increases radiation sensitivity
Abstract
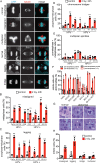
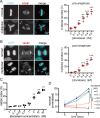
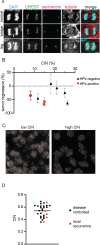
Continuous chromosome missegregation over successive mitotic divisions, known as chromosomal instability (CIN), is common in cancer. Increasing CIN above a maximally tolerated threshold leads to cell death due to loss of essential chromosomes. Here, we show in two tissue contexts that otherwise isogenic cancer cells with higher levels of CIN are more sensitive to ionizing radiation, which itself induces CIN. CIN also sensitizes HPV-positive and HPV-negative head and neck cancer patient derived xenograft (PDX) tumors to radiation. Moreover, laryngeal cancers with higher CIN prior to treatment show improved response to radiation therapy. In addition, we reveal a novel mechanism of radiosensitization by docetaxel, a microtubule stabilizing drug commonly used in combination with radiation. Docetaxel causes cell death by inducing CIN due to abnormal multipolar spindles rather than causing mitotic arrest, as previously assumed. Docetaxel-induced CIN, rather than mitotic arrest, is responsible for the enhanced radiation sensitivity observed in vitro and in vivo, challenging the mechanistic dogma of the last 40 years. These results implicate CIN as a potential biomarker and inducer of radiation response, which could provide valuable cancer therapeutic opportunities.
Statement of significance: Cancer cells and laryngeal tumors with higher chromosome missegregation rates are more sensitive to radiation therapy, supporting chromosomal instability as a promising biomarker of radiation response.
Keywords: Mad1; cervical cancer; docetaxel; head and neck cancer; mitosis.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Weaver BAA, Cleveland DW. Does aneuploidy cause cancer? Curr Opin Cell Biol. 2006;18:658–67. - PubMed
-
- Mitelman F.E., Johansson B. & Mertens F. 2017. Available from: (http://cgap.nci.nih.gov/Chromosomes/Mitelman
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
