Leveraging oncology collaborative networks and biomedical informatics data resources to rapidly recruit and enroll rural residents into oncology quality of life clinical trials
- PMID: 39345703
- PMCID: PMC11428118
- DOI: 10.1017/cts.2024.576
Leveraging oncology collaborative networks and biomedical informatics data resources to rapidly recruit and enroll rural residents into oncology quality of life clinical trials
Abstract
Purpose: This study assesses the feasibility of biomedical informatics resources for efficient recruitment of rural residents with cancer to a clinical trial of a quality-of-life (QOL) mobile app. These resources have the potential to reduce costly, time-consuming, in-person recruitment methods.
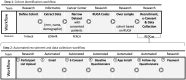
Methods: A cohort was identified from the electronic health record data repository and cross-referenced with patients who consented to additional research contact. Rural-urban commuting area codes were computed to identify rurality. Potential participants were emailed study details, screening questions, and an e-consent link via REDCap. Consented individuals received baseline questionnaires automatically. A sample minimum of n = 80 [n = 40 care as usual (CAU) n = 40 mobile app intervention] was needed.
Results: N = 1298 potential participants (n = 365 CAU; n = 833 intervention) were screened for eligibility. For CAU, 68 consented, 67 completed baseline questionnaires, and 54 completed follow-up questionnaires. For intervention, 100 consented, 97 completed baseline questionnaires, and 58 completed follow-up questionnaires. The CAU/intervention reached 82.5%/122.5% of the enrollment target within 2 days. Recruitment and retention rates were 15.3% and 57.5%, respectively. The mean age was 59.5 ± 13.5 years. The sample was 65% women, 20% racial/ethnic minority, and 35% resided in rural areas.
Conclusion: These results demonstrate that biomedical informatics resources can be highly effective in recruiting for cancer QOL research. Precisely identifying individuals likely to meet inclusion criteria who previously indicated interest in research participation expedited recruitment. Participants completed the consent and baseline questionnaires with zero follow-up contacts from the research team. This low-touch, repeatable process may be highly effective for multisite clinical trials research seeking to include rural residents.
Keywords: Cancer; informatics tools; rural health; subject recruitment; supportive oncology.
© The Author(s) 2024.
Conflict of interest statement
G.J.W. is chairman of the ORIEN Steering Committee. G.J.W. and ORIEN were not involved in developing the research question, determining the research methodology, or conducting the analyses. ORIEN did not provide financial support for this study. H.A.D., A.A.H., L.S.J., K.N., A.T.S., J.S., and S.G.W. have no competing interests.
Figures

Similar articles
-
Digital Global Recruitment for Women's Health Research: Cross-sectional Study.JMIR Form Res. 2022 Sep 14;6(9):e39046. doi: 10.2196/39046. JMIR Form Res. 2022. PMID: 35969168 Free PMC article.
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
Electronic Health Record-Based Recruitment and Retention and Mobile Health App Usage: Multisite Cohort Study.J Med Internet Res. 2022 Jun 10;24(6):e34191. doi: 10.2196/34191. J Med Internet Res. 2022. PMID: 35687400 Free PMC article.
-
Facilitators and barriers to pediatric clinical trial recruitment and retention in rural and community settings: A scoping review of the literature.Clin Transl Sci. 2022 Apr;15(4):838-853. doi: 10.1111/cts.13220. Epub 2022 Jan 21. Clin Transl Sci. 2022. PMID: 35037409 Free PMC article.
-
Telephone interventions for symptom management in adults with cancer.Cochrane Database Syst Rev. 2020 Jun 2;6(6):CD007568. doi: 10.1002/14651858.CD007568.pub2. Cochrane Database Syst Rev. 2020. PMID: 32483832 Free PMC article.
Cited by
-
Training, Validating, and Testing Machine Learning Prediction Models for Endometrial Cancer Recurrence.JCO Precis Oncol. 2025 May;9:e2400859. doi: 10.1200/PO-24-00859. Epub 2025 May 5. JCO Precis Oncol. 2025. PMID: 40324114 Free PMC article.
References
-
- Charlton M, Schlichting J, Chioreso C, Ward M, Vikas P. Challenges of rural cancer care in the United States. Oncology (Williston Park). 2015;29(9):633–640. - PubMed
-
- McPhee NJ, Nightingale CE, Harris SJ, Segelov E, Ristevski E. Barriers and enablers to cancer clinical trial participation and initiatives to improve opportunities for rural cancer patients: a scoping review. Clin Trials. 2022;19(4):464–476. - PubMed
-
- Yabroff KR, Han X, Zhao J, Nogueira L, Jemal A. Rural cancer disparities in the United States: a multilevel framework to improve access to care and patient outcomes. JCO Oncol Pract. 2020;16(7):409–413. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
