Degradable polyolefins prepared by integration of disulfides into metathesis polymerizations with 3,6-dihydro-1,2-dithiine
- PMID: 39345767
- PMCID: PMC11426189
- DOI: 10.1039/d4sc04468a
Degradable polyolefins prepared by integration of disulfides into metathesis polymerizations with 3,6-dihydro-1,2-dithiine
Abstract
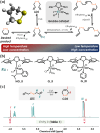
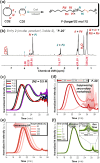
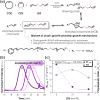
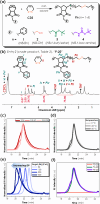
Disulfide-containing polyolefins were synthesized by ring-opening metathesis polymerization (ROMP) of the 6-membered disulfide-containing cyclic olefin, 3,6-dihydro-1,2-dithiine, which was prepared by ring-closing metathesis of diallyl disulfide. This approach facilitated the production of disulfide-containing unsaturated polyolefins as copolymers with disulfide monomer units embedded within a poly(cyclooctene) or poly(norbornene) framework. The incorporation of disulfides into the polymer backbone imparts redox responsiveness and enables polymer degradation via chemical reduction or thiol-disulfide exchange. This ROMP copolymerization strategy yielded both linear polyolefins, as well as bottlebrush polymers, with degradable segments, thereby broadening the scope of responsive polymer architectures synthesized by ROMP.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Synthesis of Degradable Polyolefins Bearing Disulfide Units via Metathesis Copolymerization.Polymers (Basel). 2023 Jul 20;15(14):3101. doi: 10.3390/polym15143101. Polymers (Basel). 2023. PMID: 37514489 Free PMC article.
-
Degradable polymers via olefin metathesis polymerization.Prog Polym Sci. 2021 Sep;120:101427. doi: 10.1016/j.progpolymsci.2021.101427. Epub 2021 Jun 7. Prog Polym Sci. 2021. PMID: 38666185 Free PMC article.
-
Tandem Ring-Opening Metathesis - Vinyl Insertion Polymerization: Fundamentals and Application to Functional Polyolefins.Macromol Rapid Commun. 2017 Mar;38(6). doi: 10.1002/marc.201600672. Epub 2017 Feb 7. Macromol Rapid Commun. 2017. PMID: 28169470 Review.
-
Tailored silyl ether monomers enable backbone-degradable polynorbornene-based linear, bottlebrush and star copolymers through ROMP.Nat Chem. 2019 Dec;11(12):1124-1132. doi: 10.1038/s41557-019-0352-4. Epub 2019 Oct 28. Nat Chem. 2019. PMID: 31659310 Free PMC article.
-
Grubbs' and Schrock's Catalysts, Ring Opening Metathesis Polymerization and Molecular Brushes-Synthesis, Characterization, Properties and Applications.Polymers (Basel). 2019 Feb 11;11(2):298. doi: 10.3390/polym11020298. Polymers (Basel). 2019. PMID: 30960282 Free PMC article. Review.
References
-
- Paxton N. C. Allenby M. C. Lewis P. M. Woodruff M. A. Biomedical applications of polyethylene. Eur. Polym. J. 2019;118:412–428. doi: 10.1016/j.eurpolymj.2019.05.037. - DOI
-
- Jubinville D. Esmizadeh E. Saikrishnan S. Tzoganakis C. Mekonnen T. A comprehensive review of global production and recycling methods of polyolefin (PO) based products and their post-recycling applications. Sustainable Mater. Technol. 2020;25:e00188. doi: 10.1016/j.susmat.2020.e00188. - DOI
-
- Yin S. Tuladhar R. Shi F. Shanks R. A. Combe M. Collister T. Mechanical reprocessing of polyolefin waste: A review. Polym. Eng. Sci. 2015;55:2899–2909. doi: 10.1002/pen.24182. - DOI
-
- Yeung C. W. S. Teo J. Y. Q. Loh X. J. Lim J. Y. C. Polyolefins and Polystyrene as Chemical Resources for a Sustainable Future: Challenges, Advances, and Prospects. ACS Mater. Lett. 2021;3:1660–1676. doi: 10.1021/acsmaterialslett.1c00490. - DOI

