Chlorquinaldol Alleviates Lung Fibrosis in Mice by Inhibiting Fibroblast Activation through Targeting Methionine Synthase Reductase
- PMID: 39345816
- PMCID: PMC11428390
- DOI: 10.1021/acscentsci.4c00798
Chlorquinaldol Alleviates Lung Fibrosis in Mice by Inhibiting Fibroblast Activation through Targeting Methionine Synthase Reductase
Abstract
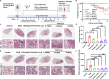
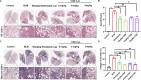
Idiopathic pulmonary fibrosis (IPF) is a progressive interstitial lung disease with limited treatment options. Thus, it is essential to investigate potential druggable targets to improve IPF treatment outcomes. By screening a curated library of 201 small molecules, we have identified chlorquinaldol, a known antimicrobial drug, as a potential antifibrotic agent. Functional analyses have demonstrated that chlorquinaldol effectively inhibits the transition of fibroblasts to myofibroblasts in vitro and mitigates bleomycin-induced pulmonary fibrosis in mice. Using a mass spectrometry-based drug affinity responsive target stability strategy, we revealed that chlorquinaldol inhibited fibroblast activation by directly targeting methionine synthase reductase (MTRR). Decreased MTRR expression was associated with IPF patients, and its reduced expression in vitro promoted extracellular matrix deposition. Mechanistically, chlorquinaldol bound to the valine residue (Val-467) in MTRR, activating the MTRR-mediated methionine cycle. This led to increased production of methionine and s-adenosylmethionine, counteracting the fibrotic effect. In conclusion, our findings suggest that chlorquinaldol may serve as a novel antifibrotic medication, with MTRR-mediated methionine metabolism playing a critical role in IPF development. Therefore, targeting MTRR holds promise as a therapeutic strategy for pulmonary fibrosis.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
CD148 Deficiency in Fibroblasts Promotes the Development of Pulmonary Fibrosis.Am J Respir Crit Care Med. 2021 Aug 1;204(3):312-325. doi: 10.1164/rccm.202008-3100OC. Am J Respir Crit Care Med. 2021. PMID: 33784491 Free PMC article.
-
Biochanin-A ameliorates pulmonary fibrosis by suppressing the TGF-β mediated EMT, myofibroblasts differentiation and collagen deposition in in vitro and in vivo systems.Phytomedicine. 2020 Nov;78:153298. doi: 10.1016/j.phymed.2020.153298. Epub 2020 Aug 1. Phytomedicine. 2020. PMID: 32781391 Free PMC article.
-
Targeting of TAM Receptors Ameliorates Fibrotic Mechanisms in Idiopathic Pulmonary Fibrosis.Am J Respir Crit Care Med. 2018 Jun 1;197(11):1443-1456. doi: 10.1164/rccm.201707-1519OC. Am J Respir Crit Care Med. 2018. PMID: 29634284 Free PMC article.
-
The heterodimer S100A8/A9 is a potent therapeutic target for idiopathic pulmonary fibrosis.J Mol Med (Berl). 2021 Jan;99(1):131-145. doi: 10.1007/s00109-020-02001-x. Epub 2020 Nov 9. J Mol Med (Berl). 2021. PMID: 33169236
-
Inositol possesses antifibrotic activity and mitigates pulmonary fibrosis.Respir Res. 2023 May 16;24(1):132. doi: 10.1186/s12931-023-02421-6. Respir Res. 2023. PMID: 37194070 Free PMC article.
Cited by
-
Metabolic dysregulation in pulmonary fibrosis: insights into amino acid contributions and therapeutic potential.Cell Death Discov. 2025 Aug 27;11(1):411. doi: 10.1038/s41420-025-02715-2. Cell Death Discov. 2025. PMID: 40858556 Free PMC article. Review.
References
-
- Brown K. K.; Martinez F. J.; Walsh S. L. F.; Thannickal V. J.; Prasse A.; Schlenker-Herceg R.; Goeldner R. G.; Clerisme-Beaty E.; Tetzlaff K.; Cottin V.; Wells A. U. The natural history of progressive fibrosing interstitial lung diseases. Eur. Respir. J. 2020, 55 (6), 2000085.10.1183/13993003.00085-2020. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
