Discovery, development and optimisation of a novel frog antimicrobial peptide with combined mode of action against drug-resistant bacteria
- PMID: 39345903
- PMCID: PMC11437748
- DOI: 10.1016/j.csbj.2024.09.006
Discovery, development and optimisation of a novel frog antimicrobial peptide with combined mode of action against drug-resistant bacteria
Abstract
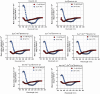
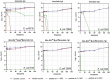
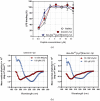
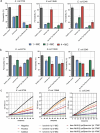
Antimicrobial peptides (AMP) have emerged as promising candidates for addressing the clinical challenges posed by the rapid evolution of antibiotic-resistant microorganisms. Brevinins, a representative frog-derived AMP family, exhibited broad-spectrum antimicrobial activities, attacking great attentions in previous studies. However, their strong haemolytic activity and cytotoxicity, greatly limit their further development. In this work, we identified and characterised a novel brevinin-1 peptide, brevinin-1pl, from the skin secretions of the northern leopard frog, Rana pipiens. Like many brevinins, brevinin-1pl also displayed strong haemolytic activity, resulting in a lower therapeutic index. We employed several bioinformatics tools to analyse the structure and potential membrane interactions of brevinin-1pl, leading to a series of modifications. Among these analogues, des-Ala16-[Lys4]brevinin-1pl exhibited great enhanced therapeutic efficacy in both in vitro and in vivo tests, particularly against some antibiotics-resistant Escherichia coli strains. Mechanistic studies suggest that des-Ala16-[Lys4]brevinin-1pl may exert bactericidal effects through multiple mechanisms, including membrane disruption and DNA binding. Consequently, des-Ala16-[Lys4]brevinin-1pl holds promise as a candidate for the treatment of drug-resistant Escherichia coli infections.
Keywords: Antimicrobial peptide; Brevinin-1; DNA binding; Drug-resistant; LPS binding.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



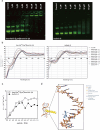
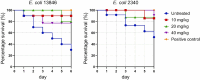
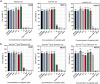
Similar articles
-
Engineering of antimicrobial peptide Brevinin-1pl: arginine, lysine, and histidine substitutions enhance antimicrobial-anticancer efficacy with reduced cytotoxicity.Front Chem. 2025 May 19;13:1579097. doi: 10.3389/fchem.2025.1579097. eCollection 2025. Front Chem. 2025. PMID: 40458657 Free PMC article.
-
Evaluating the Bioactivity of a Novel Broad-Spectrum Antimicrobial Peptide Brevinin-1GHa from the Frog Skin Secretion of Hylarana guentheri and Its Analogues.Toxins (Basel). 2018 Oct 13;10(10):413. doi: 10.3390/toxins10100413. Toxins (Basel). 2018. PMID: 30322120 Free PMC article.
-
Brevinin-2GHk from Sylvirana guentheri and the Design of Truncated Analogs Exhibiting the Enhancement of Antimicrobial Activity.Antibiotics (Basel). 2020 Feb 14;9(2):85. doi: 10.3390/antibiotics9020085. Antibiotics (Basel). 2020. PMID: 32075067 Free PMC article.
-
Biological Properties, Current Applications and Potential Therapeautic Applications of Brevinin Peptide Superfamily.Int J Pept Res Ther. 2019;25(1):39-48. doi: 10.1007/s10989-018-9723-8. Epub 2018 Jun 5. Int J Pept Res Ther. 2019. PMID: 32214928 Free PMC article. Review.
-
An overview of Brevinin superfamily: structure, function and clinical perspectives.Adv Exp Med Biol. 2014;818:197-212. doi: 10.1007/978-1-4471-6458-6_10. Adv Exp Med Biol. 2014. PMID: 25001538 Free PMC article. Review.
Cited by
-
Enhancing the selectivity and conditional sensitivity of an antimicrobial peptide through cleavage simulations and homoarginine incorporation to combat drug-resistant bacteria.Sci Rep. 2025 Jul 1;15(1):21798. doi: 10.1038/s41598-025-06522-8. Sci Rep. 2025. PMID: 40594393 Free PMC article.
-
Engineering of antimicrobial peptide Brevinin-1pl: arginine, lysine, and histidine substitutions enhance antimicrobial-anticancer efficacy with reduced cytotoxicity.Front Chem. 2025 May 19;13:1579097. doi: 10.3389/fchem.2025.1579097. eCollection 2025. Front Chem. 2025. PMID: 40458657 Free PMC article.
References
-
- Zou W., Sun R., Yao A., Zhou M., Chen X., Ma C., et al. A promising antibiotic candidate, brevinin-1 analogue 5R, against drug-resistant bacteria, with insights into its membrane-targeting mechanism. Comput Struct Biotechnol J. 2023;21:5719–5737. doi: 10.1016/j.csbj.2023.11.031. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
