In silico directed evolution of Anabas testudineus AtMP1 antimicrobial peptide to improve in vitro anticancer activity
- PMID: 39346049
- PMCID: PMC11439379
- DOI: 10.7717/peerj.17894
In silico directed evolution of Anabas testudineus AtMP1 antimicrobial peptide to improve in vitro anticancer activity
Abstract
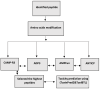
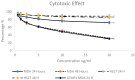
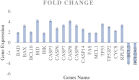
Various studies have demonstrated that directed evolution is a powerful tool in enhancing protein properties. In this study, directed evolution was used to enhance the efficacy of synthesised Anabas testudineus AtMP1 antimicrobial peptides (AMPs) in inhibiting the proliferation of cancer cells. The modification of antimicrobial peptides (AMPs) and prediction of peptide properties using bioinformatic tools were carried out using four databases, including ADP3, CAMP-R3, AMPfun, and ANTICP. One modified antimicrobial peptide (AMP), ATMP6 (THPPTTTTTTTTTTTTTAAPARTT), was chosen based on its projected potent anticancer effect, taking into account factors such as amino acid length, net charge, anticancer activity score, and hydrophobicity. The selected AMPs were subjected to study in deep-learning databases, namely ToxIBTL and ToxinPred2, to predict their toxicity. Furthermore, the allergic properties of these antimicrobial peptides (AMPs) were verified by utilising AllerTOP and AllergenFP. Based on the results obtained from the database study, it was projected that antimicrobial peptides (AMPs) demonstrate a lack of toxicity towards human cells that is indicative of the broader population. After 48 hours of incubation, the IC50 values of ATMP6 against the HS27 and MDA-MB-231 cell lines were found to be 48.03 ± 0.013 µg/ml and 7.52 ± 0.027 µg/ml, respectively. The IC50 values of the original peptide ATMP1 against the MDA-MB-231 and HS27 cell lines were determined to be 59.6 ± 0.14 µg/ml and 8.25 ± 0.14 µg/ml, respectively, when compared. Furthermore, the results indicated that the injection of ATMP6 induced apoptosis in the MDA-MB-231 cell lines. The present investigation has revealed new opportunities for advancing novel targeted peptide therapeutics to tackle cancer.
Keywords: Anabas testudineus; Antimicrobial peptide; Breast cancer; Directed evolution; Peptide drug.
©2024 Fazry et al.
Conflict of interest statement
The authors declare there are no competing interests except Choy-Theng Loh is employed by Hangzhou Foreseebio Biotechnology Co., Ltd and being appointed as adjunct Faculty Member at INTI International University. All the work done by was Choy-Theng Loh was purely academic based.
Figures

References
-
- Alijani Ardeshir R, Rastgar S, Morakabati P, Mojiri-Forushani H, Movahedinia A, Salati AP. Selective induced apoptosis and cell cycle arrest in MCF7 and LNCap cell lines by skin mucus from round goby (Neogobius melanostomus) and common carp (Cyprinus carpio) through P53 expression. Cytotechnology. 2020;72:367–376. doi: 10.1007/s10616-020-00383-x. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

