Construction and validation of co-expression vector for rice alpha tubulin and microtubule associated protein respectively fused with fluorescent proteins
- PMID: 39346063
- PMCID: PMC11439384
- DOI: 10.7717/peerj.18118
Construction and validation of co-expression vector for rice alpha tubulin and microtubule associated protein respectively fused with fluorescent proteins
Abstract
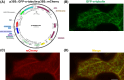
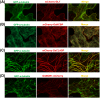
Microtubule (MT) consists of α-tubulin and β-tubulin. The dynamic instability regulated by various microtubule associated proteins (MAPs) is essential for MT functions. To analyze the interaction between tubulin/MT and MAP in vivo, we usually need tubulin and MAP co-expressed. Here, we constructed a dual-transgene vector expressing rice (Oryza sativa) α-tubulin and MAP simultaneously. To construct this vector, plant expression vector pCambia1301 was used as the plasmid backbone and Gibson assembly cloning technology was used. We first fused and cloned the GFP fragment, α-tubulin open reading frame (ORF), and NOS terminator into the vector pCambia1301 to construct the p35S::GFP-α-tubulin vector that expressed GFP-α-tubulin fusion protein. Subsequently, we fused and cloned the CaMV 35S promoter, mCherry fragment, and NOS terminator into the p35S::GFP-α-tubulin vector to generate the universal dual-transgene expression vector (p35S::GFP-α-tubulin-p35S::mCherry vector). With the p35S::GFP-α-tubulin-p35S::mCherry vector, MAP ORF can be cloned into the site of 5' or 3' terminus of mCherry to co-express GFP-α-tubulin and MAP-mCherry/mCherry-MAP. To validate the availability and universality of the dual-transgene expression vector, a series of putative rice MAP genes including GL7, OsKCBP, OsCLASP, and OsMOR1 were cloned into the vector respectively, transformed into Agrobacterium tumefaciens strain, and expressed in Nicotiana benthamiana leaves. The results indicated that all of the MAPs were co-expressed with α-tubulin and localized to MTs, validating the availability and universality of the vector and that GL7, OsKCBP, OsCLASP, and OsMOR1 might be MAPs. The application of the co-expression vector constructed by us would facilitate studies on the interaction between tubulin/MT and MAP in tobacco transient expression systems or transgenic rice.
Keywords: Co-expression vector; Fluorescent protein; Microtubule; Microtubule associated protein; Rice; Tubulin.
© 2024 Xu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Aher A, Kok M, Sharma A, Rai A, Olieric N, Rodriguez-Garcia R, Katrukha EA, Weinert T, Olieric V, Kapitein LC, Steinmetz MO, Dogterom M, Akhmanova A. CLASP suppresses microtubule catastrophes through a single TOG domain. Developmental Cell. 2018;46(1):40–58. doi: 10.1016/j.devcel.2018.05.032. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

