Strategies for oxidative synthesis of N-triflyl sulfoximines
- PMID: 39346519
- PMCID: PMC11427871
- DOI: 10.1039/d4ra04992f
Strategies for oxidative synthesis of N-triflyl sulfoximines
Abstract
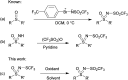
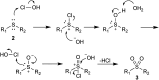
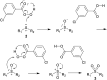
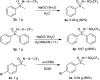
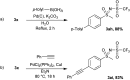
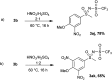
The oxidation of various structurally different N-trifluoromethylthio sulfoximines was investigated using different oxidizing agents and conditions. Mono- and disubstituted phenyl methyl and phenyl cyclopropyl N-trifluoromethylthio sulfoximines were oxidized with NaOCl·5H2O in water, while sterically hindered substrates bearing bulkier alkyl chains or two phenyl rings required the addition of MeCN to the reaction mixture. Chloro-, bromo-, and cyano-substituted substrates, as well as substrates bearing the benzyl groups, required a completely different approach using m-CPBA in DCM. Each method was tested on a gram-scale, with almost no difference in yield or reaction profile. The methods were also tested on N-p-tolylthio sulfoximine where successful oxidation to the corresponding sulfone derivative was observed. Finally, the N-triflyl sulfoximines acquired in the oxidations were examined in terms of stability and reactivity in Suzuki-Miyaura and Sonogashira coupling reactions, as well as many others. The selective mono- and dinitration of 4-methoxyphenyl N-triflyl sulfoximine was demonstrated by using nitric and sulfuric acid. N-triflyl sulfoximines were found to be stable in concentrated aqueous NaOH and HCl solutions and at elevated temperatures.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

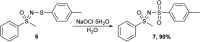
References
-
- Lücking U. Org. Chem. Front. 2019;6:1319–1324.
-
- Frings M. Bolm C. Blum A. Gnamm C. Eur. J. Med. Chem. 2017;126:225–245. - PubMed
-
- Han Y. Xing K. Zhang J. Tong T. Shi Y. Cao H. Yu H. Zhang Y. Liu D. Zhao L. Eur. J. Med. Chem. 2021;209:112885. - PubMed
-
- Mäder P. Kattner L. J. Med. Chem. 2020;63:14243–14275. - PubMed
-
- Lücking U. Chem.–Eur. J. 2022;28:e202201993. - PubMed
LinkOut - more resources
Full Text Sources

