ROCK inhibitor enhances mitochondrial transfer via tunneling nanotubes in retinal pigment epithelium
- PMID: 39346535
- PMCID: PMC11426248
- DOI: 10.7150/thno.96508
ROCK inhibitor enhances mitochondrial transfer via tunneling nanotubes in retinal pigment epithelium
Abstract
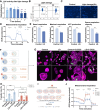
Rationale: Tunnel nanotube (TNT)-mediated mitochondrial transport is crucial for the development and maintenance of multicellular organisms. Despite numerous studies highlighting the significance of this process in both physiological and pathological contexts, knowledge of the underlying mechanisms is still limited. This research focused on the role of the ROCK inhibitor Y-27632 in modulating TNT formation and mitochondrial transport in retinal pigment epithelial (RPE) cells. Methods: Two types of ARPE19 cells (a retinal pigment epithelial cell line) with distinct mitochondrial fluorescently labeled, were co-cultured and treated with ROCK inhibitor Y-27632. The formation of nanotubes and transport of mitochondria were assessed through cytoskeletal staining and live cell imaging. Mitochondrial dysfunction was induced by light damage to establish a model, while mitochondrial function was evaluated through measurement of oxygen consumption rate. The effects of Y-27632 on cytoskeletal and mitochondrial dynamics were further elucidated through detailed analysis. Results: Y-27632 treatment led to an increase in nanotube formation and enhanced mitochondrial transfer among ARPE19 cells, even following exposure to light-induced damage. Our analysis of cytoskeletal and mitochondrial distribution changes suggests that Y-27632 promotes nanotube-mediated mitochondrial transport by influencing cytoskeletal remodeling and mitochondrial movement. Conclusions: These results suggest that Y-27632 has the ability to enhance mitochondrial transfer via tunneling nanotubes in retinal pigment epithelium, and similarly predict that ROCK inhibitor can fulfill its therapeutic potential through promoting mitochondrial transport in the retinal pigment epithelium in the future.
Keywords: ARPE19; Y-27632; cytoskeletal remodeling; light damage; mitochondrial transfer; nanotubes.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Li Q, Wang M, Liu L. The role of exosomes in the stemness maintenance and progression of acute myeloid leukemia. Biochem Pharmacol. 2023;212:115539. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

