Single-cell RNA-Seq analysis of molecular changes during radiation-induced skin injury: the involvement of Nur77
- PMID: 39346541
- PMCID: PMC11426238
- DOI: 10.7150/thno.100417
Single-cell RNA-Seq analysis of molecular changes during radiation-induced skin injury: the involvement of Nur77
Abstract
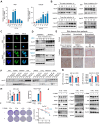
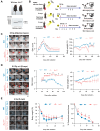
Introduction: Ionizing radiation has been widely used in industry, medicine, military and agriculture. Radiation-induced skin injury is a significant concern in the context of radiotherapy and accidental exposure to radiation. The molecular changes at the single-cell level and intercellular communications during radiation-induced skin injury are not well understood. Methods: This study aims to illustrate this information in a murine model and human skin samples from a radiation accident using single-cell RNA sequencing (scRNA-Seq). We further characterize the functional significance of key molecule, which may provide a potential therapeutic target. ScRNA-Seq was performed on skin samples from a nuclear accident patient and rats exposed to ionizing radiation. Bioinformatic tools were used to analyze the cellular heterogeneity and preferential mRNAs. Comparative analysis was performed to identify dysregulated pathways, regulators, and ligand-receptor interactions in fibroblasts. The function of key molecule was validated in skin cells and in three mouse models of radiation-induced skin injury. Results: 11 clusters in human skin and 13 clusters of cells in rat skin were depicted respectively. Exposure to ionizing radiation caused changes in the cellular population (upregulation of fibroblasts and endothelial cells, downregulation of keratinocytes). Fibroblasts and keratinocytes possessed the most interaction pairs with other cell lineages. Among the five DEGs common to human and rat skins, Nur77 was highly expressed in fibroblasts, which mediated radiosensitivity by cell apoptosis and modulated crosstalk between macrophages, keratinocytes and endothelial cells in radiation-induced skin injury. In animal models, Nur77 knock-out mice (Nur77 -/-) showed more severe injury after radiation exposure than wild-type counterparts in three models of radiation-induced skin injury with complex mechanisms. Conclusion: The study reveals a single-cell transcriptional framework during radiation-induced skin injury, which provides a useful resource to uncover key events in its progression. Nur77 is a novel target in radiation-induced skin injury, which provides a potential therapeutic strategy against this disease.
Keywords: ionizing radiation; orphan nuclear receptor 77 (Nur77); radiation-induced skin injury; single-cell RNA sequencing (scRNA-Seq); skin.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Kabashima K, Honda T, Ginhoux F, Egawa G. The immunological anatomy of the skin. Nat Rev Immunol. 2019;19(1):19–30. - PubMed
-
- Wang K, Tepper JE. Radiation therapy-associated toxicity: etiology, management, and prevention. CA Cancer J Clin. 2021;71(5):437–54. - PubMed
-
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56(9):909–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

