Collagen type I PET/MRI enables evaluation of treatment response in pancreatic cancer in pre-clinical and first-in-human translational studies
- PMID: 39346545
- PMCID: PMC11426233
- DOI: 10.7150/thno.100116
Collagen type I PET/MRI enables evaluation of treatment response in pancreatic cancer in pre-clinical and first-in-human translational studies
Abstract
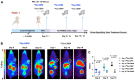
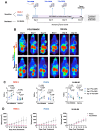
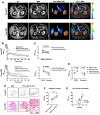
Pancreatic ductal adenocarcinoma (PDAC) is an invasive and rapidly progressive malignancy. A major challenge in patient management is the lack of a reliable imaging tool to monitor tumor response to treatment. Tumor-associated fibrosis characterized by high type I collagen is a hallmark of PDAC, and fibrosis further increases in response to neoadjuvant chemoradiotherapy (CRT). We hypothesized that molecular positron emission tomography (PET) using a type I collagen-specific imaging probe, 68Ga-CBP8 can detect and measure changes in tumor fibrosis in response to standard treatment in mouse models and patients with PDAC. Methods: We evaluated the specificity of 68Ga-CBP8 PET to tumor collagen and its ability to differentiate responders from non-responders based on the dynamic changes of fibrosis in nude mouse models of human PDAC including FOLFIRNOX-sensitive (PANC-1 and PDAC6) and FOLFIRINOX-resistant (SU.86.86). Next, we demonstrated the specificity and sensitivity of 68Ga-CBP8 to the deposited collagen in resected human PDAC and pancreas tissues. Eight male participant (49-65 y) with newly diagnosed PDAC underwent dynamic 68Ga-CBP8 PET/MRI, and five underwent follow up 68Ga-CBP8 PET/MRI after completing standard CRT. PET parameters were correlated with tumor collagen content and markers of response on histology. Results: 68Ga-CBP8 showed specific binding to PDAC compared to non-binding 68Ga-CNBP probe in two mouse models of PDAC using PET imaging and to resected human PDAC using autoradiography (P < 0.05 for all comparisons). 68Ga-CBP8 PET showed 2-fold higher tumor signal in mouse models following FOLFIRINOX treatment in PANC-1 and PDAC6 models (P < 0.01), but no significant increase after treatment in FOLFIRINOX resistant SU.86.86 model. 68Ga-CBP8 binding to resected human PDAC was significantly higher (P < 0.0001) in treated versus untreated tissue. PET/MRI of PDAC patients prior to CRT showed significantly higher 68Ga-CBP8 uptake in tumor compared to pancreas (SUVmean: 2.35 ± 0.36 vs. 1.99 ± 0.25, P = 0.036, n = 8). PET tumor values significantly increased following CRT compared to untreated tumors (SUVmean: 2.83 ± 0.30 vs. 2.25 ± 0.41, P = 0.01, n = 5). Collagen deposition significantly increased in response to CRT (59 ± 9% vs. 30 ± 9%, P=0.0005 in treated vs. untreated tumors). Tumor and pancreas collagen content showed a positive direct correlation with SUVmean (R2 = 0.54, P = 0.0007). Conclusions: This study demonstrates the specificity of 68Ga-CBP8 PET to tumor type I collagen and its ability to differentiate responders from non-responders based on the dynamic changes of fibrosis in PDAC. The results highlight the potential use of collagen PET as a non-invasive tool for monitoring response to treatment in patients with PDAC.
Keywords: PET imaging; fibrosis; pancreatic cancer; treatment response; type I collagen.
© The author(s).
Conflict of interest statement
Competing Interests: PC has equity in and is a consultant to Collagen Medical LLC, has equity in Reveal Pharmaceuticals Inc., and has research support from Transcode Therapeutics and Pliant Therapeutics. PC is a co-inventor of US Patent 10,471,162 which covers 68Ga-CBP8 and is assigned to the General Hospital Corporation. SE has research support from Sofie Biosciences, Telix, and Novartis Pharmaceuticals. ARP has held Equity in C2i Genomics, XGenomes, Cadex, Vionix and Parithera. In the last 36 months, she has served as an advisor/consultant for Eli Lilly, Mirati, Pfizer, Inivata, Biofidelity, Checkmate Pharmaceuticals, FMI, Guardant, Abbvie, Bayer, Delcath, Taiho, CVS, Value Analytics Lab, Seagen, Saga, AZ, Scare Inc, Illumina, Taiho, Hookipa, Kahar Medical, Xilio Therapeutics, Sirtex, Takeda, and Science For America. She receives fees from Up to Date. She has received travel fees from Karkinos Healthcare. She has been on the DSMC for a Roche study and on the Steering Committee for Exilixis. She has received research funding to the Institution from PureTech, PMV Pharmaceuticals, Plexxicon, Takeda, BMS, Mirati, Novartis, Erasca, Genentech, Daiichi Sankyo, Syndax, Revolution Medicine and Parthenon. UM is a co-founder, shareholder, and consultant (Scientific Advisory Board) of CytoSite BioPharma. TSH is a consultant for Synthetic Biologics, Novocure, Boston Scientific, Neogenomics, Merck, GSK, NextCure, serves on the advisory board of PanTher Therapeutics (Equity), and Lustgarten Foundation, and has received research funding from Taiho, Astra-Zeneca, BMS, GSK, ItraOp and Ipsen. GMB has sponsored research agreements through her institution with: Olink Proteomics, Teiko Bio, InterVenn Biosciences, Palleon Pharmaceuticals. She served on advisory boards for Iovance, Merck, Nektar Therapeutics, Novartis, and Ankyra Therapeutics. She consults for Merck, InterVenn Biosciences, Iovance, and Ankyra Therapeutics. She holds equity in Ankyra Therapeutics. Other authors declare that they have no competing interests.
Figures



References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. - PubMed
-
- Seufferlein T, Bachet JB, Van Cutsem E, Rougier P, Group EGW. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii33–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

