Quantitative pulmonary pharmacokinetics of tetrandrine for SARS-CoV-2 repurposing: a physiologically based pharmacokinetic modeling approach
- PMID: 39346557
- PMCID: PMC11427368
- DOI: 10.3389/fphar.2024.1457983
Quantitative pulmonary pharmacokinetics of tetrandrine for SARS-CoV-2 repurposing: a physiologically based pharmacokinetic modeling approach
Abstract
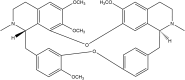
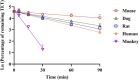
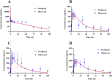
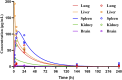
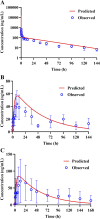
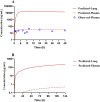
Tetrandrine (TET) has been traditionally used in China as a medication to treat silicosis and has recently demonstrated anti-SARS-CoV-2 potential in vitro. By recognizing the disparity between in vitro findings and in vivo performance, we aimed to estimate the free lung concentration of TET using a physiologically based pharmacokinetic (PBPK) model to link in vitro activity with in vivo efficacy. Comparative pharmacokinetic studies of TET were performed in rats and dogs to elucidate the pharmacokinetic mechanisms as well as discern interspecies variations. These insights facilitated the creation of an animal-specific PBPK model, which was subsequently translated to a human model following thorough validation. Following validation of the pharmacokinetic profile from a literature report on single oral dosing of TET in humans, the plasma and lung concentrations were predicted after TET administration at approved dosage levels. Finally, the antiviral efficacy of TET in humans was assessed from the free drug concentration in the lungs. Both in vivo and in vitro experiments thus confirmed that the systemic clearance of TET was primarily through hepatic metabolism. Additionally, the lysosomal capture of basic TET was identified as a pivotal factor in its vast distribution volume and heterogeneous tissue distribution, which could modulate the absorption dynamics of TET in the gastrointestinal tract. Notably, the PBPK-model-based unbound lung concentration of TET (1.67-1.74 μg/mL) at the recommended clinical dosage surpassed the in vitro threshold for anti-SARS-CoV-2 activity (EC90 = 1.52 μg/mL). Thus, a PBPK model was successfully developed to bridge the in vitro activity and in vivo target exposure of TET to facilitate its repurposing.
Keywords: drug repurpose; lysosomal trapping; physiologically based pharmacokinetic (PBPK); pulmonary exposure; tetrandrine.
Copyright © 2024 Wang, Dong, Hu, Yang, Wang, Zhang, Zhang and Zhuang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Antiviral effects and tissue exposure of tetrandrine against SARS-CoV-2 infection and COVID-19.MedComm (2020). 2023 Jan 19;4(1):e206. doi: 10.1002/mco2.206. eCollection 2023 Feb. MedComm (2020). 2023. PMID: 36699286 Free PMC article.
-
Use of physiological based pharmacokinetic modeling for cross-species prediction of pharmacokinetic and tissue distribution profiles of a novel niclosamide prodrug.Front Pharmacol. 2023 Apr 11;14:1099425. doi: 10.3389/fphar.2023.1099425. eCollection 2023. Front Pharmacol. 2023. PMID: 37113753 Free PMC article.
-
The Development of a Physiologically Based Pharmacokinetic (PBPK) Model of Andrographolide in Mice and Scaling it up to Rats, Dogs, and Humans.Curr Drug Metab. 2022;23(7):538-552. doi: 10.2174/1389200223666220628095616. Curr Drug Metab. 2022. PMID: 35762544
-
PBPK models for the prediction of in vivo performance of oral dosage forms.Eur J Pharm Sci. 2014 Jun 16;57:300-21. doi: 10.1016/j.ejps.2013.09.008. Epub 2013 Sep 21. Eur J Pharm Sci. 2014. PMID: 24060672 Review.
-
The Role of Extracellular Binding Proteins in the Cellular Uptake of Drugs: Impact on Quantitative In Vitro-to-In Vivo Extrapolations of Toxicity and Efficacy in Physiologically Based Pharmacokinetic-Pharmacodynamic Research.J Pharm Sci. 2016 Feb;105(2):497-508. doi: 10.1002/jps.24571. Epub 2016 Jan 11. J Pharm Sci. 2016. PMID: 26173749 Review.
References
-
- Arshad U., Pertinez H., Box H., Tatham L., Rajoli R. K. R., Curley P., et al. (2020). Prioritization of anti-SARS-cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics. Clin. Pharmacol. Ther. 108 (4), 775–790. 10.1002/cpt.1909 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

