The leukotriene receptor antagonist montelukast as a potential therapeutic adjuvant in multiple sclerosis - a review
- PMID: 39346564
- PMCID: PMC11427386
- DOI: 10.3389/fphar.2024.1450493
The leukotriene receptor antagonist montelukast as a potential therapeutic adjuvant in multiple sclerosis - a review
Abstract
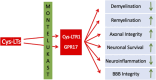
Multiple Sclerosis (MS) is a multifactorial autoimmune disease of the central nervous system (CNS). It is characterized by a heightened activation of the immune system with ensuing inflammation, demyelination and neurodegeneration with consequences such as motor, sensory, cognitive, as well as autonomic dysfunctions. While a range of immune-modulatory drugs have shown certain efficacy in alleviating pathology and symptoms, none of the currently available therapeutics regenerates the damaged CNS to restore function. There is emerging evidence for leukotrienes and leukotriene receptors being involved in the various aspects of the MS pathology including neuroinflammation and de/remyelination. Moreover, leukotriene receptor antagonists such as the asthma drug montelukast diminish inflammation and promote regeneration/remyelination. Indeed, montelukast has successfully been tested in animal models of MS and a recent retrospective case-control study suggests that montelukast treatment reduces relapses in patients with MS. Therefore, we propose montelukast as a therapeutic adjuvant to the standard immune-modulatory drugs with the potential to reduce pathology and promote structural and functional restoration. Here, we review the current knowledge on MS, its pathology, and on the potential of leukotriene receptor antagonists as therapeutics for MS.
Keywords: drug development; neuroinflammation; regeneration; remyelination; restoration.
Copyright © 2024 Pietrantonio, Serreqi, Zerbe, Svenningsson and Aigner.
Conflict of interest statement
FP, AS, and HZ are employees at IntelgenX corp., which holds patents in the use of montelukast in neurodegenerative diseases. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
Figures
References
-
- Bäck M., Dahlén S. E., Drazen J. M., Evans J. F., Serhan C. N., Shimizu T., et al. (2011). International Union of Basic and Clinical Pharmacology. LXXXIV: leukotriene receptor nomenclature, distribution, and pathophysiological functions. Pharmacol. Rev. 63 (3), 539–584. 10.1124/pr.110.004184 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources