A comparative study on the effects of biodegradable high-purity magnesium screw and polymer screw for fixation in epiphyseal trabecular bone
- PMID: 39346687
- PMCID: PMC11427752
- DOI: 10.1093/rb/rbae095
A comparative study on the effects of biodegradable high-purity magnesium screw and polymer screw for fixation in epiphyseal trabecular bone
Abstract
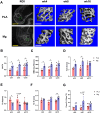
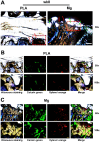
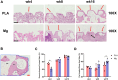
With mechanical strength close to cortical bone, biodegradable and osteopromotive properties, magnesium (Mg)-based implants are promising biomaterials for orthopedic applications. However, during the degradation of such implants, there are still concerns on the potential adverse effects such as formation of cavities, osteolytic phenomena and chronic inflammation. Therefore, to transform Mg-based implants into clinical practice, the present study evaluated the local effects of high-purity Mg screws (HP-Mg, 99.99 wt%) by comparing with clinically approved polylactic acid (PLA) screws in epiphyseal trabecular bone of rabbits. After implantation of screws at the rabbit distal femur, bone microstructural, histomorphometric and biomechanical properties were measured at various time points (weeks 4, 8 and 16) using micro-CT, histology and histomorphometry, micro-indentation and scanning electron microscope. HP-Mg screws promoted peri-implant bone ingrowth with higher bone mass (BV/TV at week 4: 0.189 ± 0.022 in PLA group versus 0.313 ± 0.053 in Mg group), higher biomechanical properties (hardness at week 4: 35.045 ± 1.000 HV in PLA group versus 51.975 ± 2.565 HV in Mg group), more mature osteocyte LCN architecture, accelerated bone remodeling process and alleviated immunoreactive score (IRS of Ram11 at week 4: 5.8 ± 0.712 in PLA group versus 3.75 ± 0.866 in Mg group) as compared to PLA screws. Furthermore, we conducted finite element analysis to validate the superiority of HP-Mg screws as orthopedic implants by demonstrating reduced stress concentration and uniform stress distribution around the bone tunnel, which led to lower risks of trabecular microfractures. In conclusion, HP-Mg screws demonstrated greater osteogenic bioactivity and limited inflammatory response compared to PLA screws in the epiphyseal trabecular bone of rabbits. Our findings have paved a promising way for the clinical application of Mg-based implants.
Keywords: epiphyseal trabecular bone; high-purity magnesium screw; macrophage; peri-implant bone quality; polylactic acid screw.
© The Author(s) 2024. Published by Oxford University Press.
Figures





Similar articles
-
Magnesium alloy based interference screw developed for ACL reconstruction attenuates peri-tunnel bone loss in rabbits.Biomaterials. 2018 Mar;157:86-97. doi: 10.1016/j.biomaterials.2017.12.007. Epub 2017 Dec 11. Biomaterials. 2018. PMID: 29248806
-
Long-term in vivo evolution of high-purity Mg screw degradation - Local and systemic effects of Mg degradation products.Acta Biomater. 2018 Apr 15;71:215-224. doi: 10.1016/j.actbio.2018.02.023. Epub 2018 Mar 17. Acta Biomater. 2018. PMID: 29505891
-
Magnesium (Mg) based interference screws developed for promoting tendon graft incorporation in bone tunnel in rabbits.Acta Biomater. 2017 Nov;63:393-410. doi: 10.1016/j.actbio.2017.09.018. Epub 2017 Sep 14. Acta Biomater. 2017. PMID: 28919510
-
A magnesium screw with optimized geometry exhibits improved corrosion resistance and favors bone fracture healing.Acta Biomater. 2024 Apr 1;178:320-329. doi: 10.1016/j.actbio.2024.03.002. Epub 2024 Mar 11. Acta Biomater. 2024. PMID: 38479677
-
In vitro and in vivo studies on the degradation of high-purity Mg (99.99wt.%) screw with femoral intracondylar fractured rabbit model.Biomaterials. 2015 Sep;64:57-69. doi: 10.1016/j.biomaterials.2015.06.031. Epub 2015 Jun 20. Biomaterials. 2015. PMID: 26117658
Cited by
-
Stress-Strain State Investigation and Ultimate Load on Femoral Implants Based on S-Type Ti6Al4V Titanium Alloy.J Funct Biomater. 2025 May 19;16(5):187. doi: 10.3390/jfb16050187. J Funct Biomater. 2025. PMID: 40422851 Free PMC article.
-
Multiscale metal-based nanocomposites for bone and joint disease therapies.Mater Today Bio. 2025 Apr 17;32:101773. doi: 10.1016/j.mtbio.2025.101773. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40290898 Free PMC article. Review.
References
-
- Oden A, McCloskey EV, Kanis JA, Harvey NC, Johansson H.. Burden of high fracture probability worldwide: secular increases 2010-2040. Osteoporos Int 2015;26:2243–8. - PubMed
-
- Adams J, Wilson N, Hurkmans E, Bakkers M, Balazova P, Baxter M, Blavnsfeldt AB, Briot K, Chiari C, Cooper C, Dragoi RG, Gabler G, Lems W, Mosor E, Pais S, Simon C, Studenic P, Tilley S, de la Torre-Aboki J, Stamm TA.. 2019 EULAR points to consider for non-physician health professionals to prevent and manage fragility fractures in adults 50 years or older. Ann Rheum Dis 2021;80:57–64. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

