Association of COX-inhibitors with cancer patients' survival under chemotherapy and radiotherapy regimens: a real-world data retrospective cohort analysis
- PMID: 39346725
- PMCID: PMC11427433
- DOI: 10.3389/fonc.2024.1433497
Association of COX-inhibitors with cancer patients' survival under chemotherapy and radiotherapy regimens: a real-world data retrospective cohort analysis
Abstract
Introduction: We conducted an extensive, sex-oriented real-world data analysis to explore the impact and safety of non-steroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors (coxibs) on cancer treatment outcomes. This is particularly relevant given the role of the COX-2/PGE2 pathway in tumor cell resistance to chemotherapy and radiotherapy.
Methods: The study applied a retrospective cohort design utilizing the TriNetX research database consisting of patients receiving cancer treatment in 2008-2022. The treated cohorts included patients who were prescribed with coxibs, aspirin or ibuprofen, while individuals in the control cohort did not receive these medicines during their cancer treatment. A 1:1 propensity score matching technique was used to balance the baseline characteristics in the treated and control cohorts. Then, Cox proportional hazards regression and logistic regression were applied to assess the mortality and morbidity risks among patient cohorts in a 5-year follow-up period.
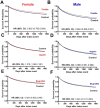
Results: Use of coxibs (HR, 0.825; 95% CI 0.792-0.859 in females and HR, 0.884; 95% CI 0.848-0.921 in males) and ibuprofen (HR, 0.924; 95% CI 0.903-0.945 in females and HR, 0.940; 95% CI 0.917-0.963 in males) were associated with improved survival. Female cancer patients receiving aspirin presented increased mortality (HR, 1.078; 95% CI 1.060-1.097), while male cancer patients also had improved survival when receiving aspirin (HR, 0.966; 95% CI 0.951-0.980). Cancer subtype specific analysis suggests coxibs and ibuprofen correlated with survival, though ibuprofen and aspirin increased emergency department visits' risk. Secondary analyses, despite limited by small cohort sizes, suggest that COX inhibition post-cancer diagnosis may benefit patients with specific cancer subtypes.
Discussion: Selective COX-2 inhibition significantly reduced mortality and emergency department visit rates. Further clinical trials are needed to determine the optimal conditions for indication of coxibs as anti-inflammatory adjuvants in cancer treatment.
Keywords: COX-inhibitors; cancer survival; chemotherapy; multiarm; multistage; non-steroidal anti-inflammatory drugs; platform; radiotherapy.
Copyright © 2024 Flausino, Ferreira, Tuan, Estevez-Diz and Chammas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Impact of SGLT2 inhibitors on survival in gastrointestinal cancer patients undergoing chemotherapy and/or radiotherapy: a real-world data retrospective cohort study.BMC Cancer. 2025 Mar 25;25(1):542. doi: 10.1186/s12885-025-13966-8. BMC Cancer. 2025. PMID: 40133838 Free PMC article.
-
Evaluation of risk profiles for gastrointestinal and cardiovascular adverse effects in nonselective NSAID and COX-2 inhibitor users: a cohort study using pharmacy dispensing data in The Netherlands.Drug Saf. 2008;31(2):143-58. doi: 10.2165/00002018-200831020-00004. Drug Saf. 2008. PMID: 18217790
-
Low-dose Aspirin, Nonsteroidal Anti-inflammatory Drugs, Selective COX-2 Inhibitors and Breast Cancer Recurrence.Epidemiology. 2016 Jul;27(4):586-93. doi: 10.1097/EDE.0000000000000480. Epidemiology. 2016. PMID: 27007644 Free PMC article.
-
Non-steroidal anti-inflammatory drug-induced cardiovascular adverse events: a meta-analysis.J Clin Pharm Ther. 2017 Feb;42(1):27-38. doi: 10.1111/jcpt.12484. Epub 2016 Dec 26. J Clin Pharm Ther. 2017. PMID: 28019014 Review.
-
Anti-inflammatory drugs in the 21st century.Subcell Biochem. 2007;42:3-27. doi: 10.1007/1-4020-5688-5_1. Subcell Biochem. 2007. PMID: 17612044 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

