Regioselective Oxidation of Tetrahydronaphthalenes to α-Tetralone Derivatives Using DDQ as Oxidizing Agent: Synthesis and Evaluation of Antibacterial and Antifungal Activities
- PMID: 39346887
- PMCID: PMC11425643
- DOI: 10.1021/acsomega.4c02130
Regioselective Oxidation of Tetrahydronaphthalenes to α-Tetralone Derivatives Using DDQ as Oxidizing Agent: Synthesis and Evaluation of Antibacterial and Antifungal Activities
Abstract
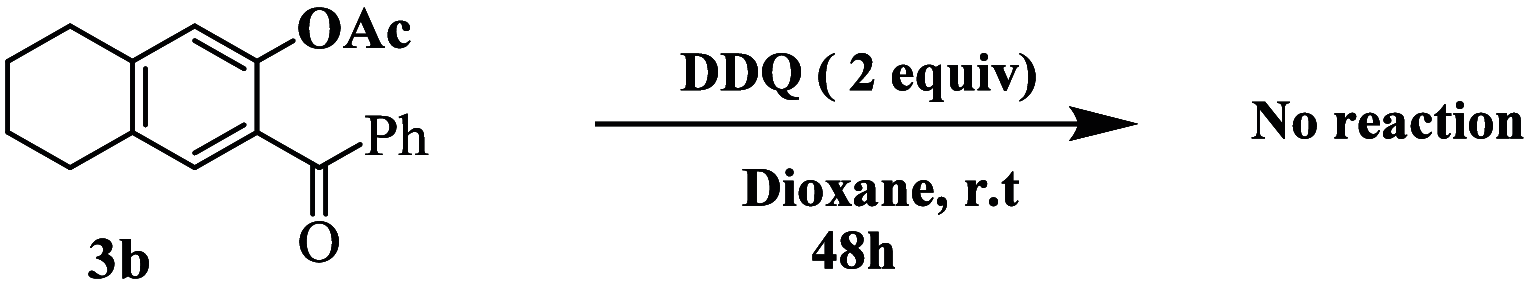
An easy and efficient approach for the synthesis of highly regioselective functionalized dihydronaphthalen-1(2H)-one family of α-tetralones from functionalized tetralone precursors which derived from Morita-Baylis-Hillman (MBH) adducts as starting substrates has been developed. The target dihydronaphthalen-1(2H)-ones are obtained through the oxidation of tetrahydronaphthalenes (THN) using DDQ as the oxidizing agent, conducted in aqueous acetic acid at reflux conditions. The yields obtained ranged from 90 to 98%. The resulting dihydronaphthalen-1(2H)-ones were evaluated for their in vitro antibacterial activity against nine Gram-positive and six Gram-negative strains. Additionally, their antifungal properties were assessed against three fungal pathogens by using the microdilution method and Biolog Phenotype Microarrays technology. Remarkably, the synthesized dihydronaphthalen-1(2H)-ones exhibited good antibacterial activity when compared to reference drugs such as vancomycin and ampicillin. Similarly, their antifungal activity is comparable to the effectiveness of the reference drugs cycloheximide and fluconazole.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



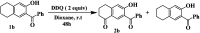
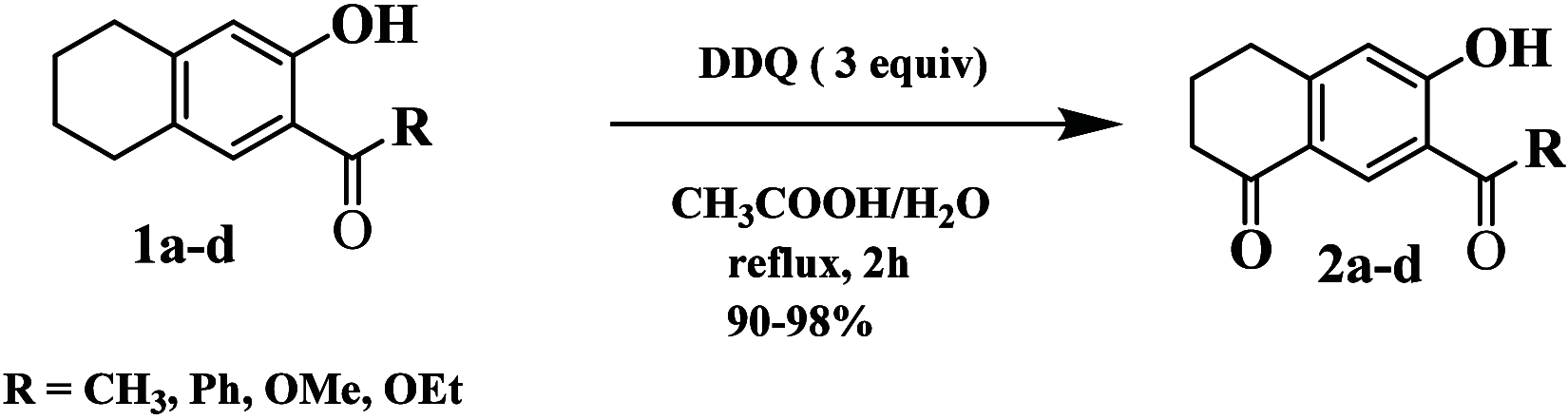
References
-
- Prasomsri T.; Galiasso Tailleur R. E.; Alvarez W. E.; Sooknoi T.; Resasco D. E. Conversion of 1- and 2-Tetralone Over HY Zeolite. Catal. Lett. 2010, 135, 226–232. 10.1007/s10562-010-0308-1. - DOI
-
- Souza L. G.; de O. Domingos J. L.; de A. Fernandes T.; Renno M. N.; Sansano J. M.; Najera C.; Costa P. R.R. Enantio selective electrophilic fluorination of α-aryl-tetralonesusing a preparation of N-fluoroammoniumsalts of cinchonine. J. Org. Chem. 2019, 217, 72–79. 10.1016/j.jfluchem.2018.11.007. - DOI
-
- Labadie G. R.; Cravero R. M.; Gonzalez-Sierra M. Studies of Birch Reductive Alkylation of Substituted α-Tetralones. Synth. Commun. 2000, 30, 4065–4079. 10.1080/00397910008087022. - DOI
-
- Labadie G. R.; Estiu G. L.; Cravero R. M.; Sierra M. G. Decompositionmechanism of Birch alkylation products of α-tetralones. J. Mol. Struct. 2003, 635, 173–182. 10.1016/S0166-1280(03)00407-X. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
