Neu1 deficiency and fibrotic lymph node microenvironment lead to imbalance in M1/M2 macrophage polarization
- PMID: 39346907
- PMCID: PMC11427323
- DOI: 10.3389/fimmu.2024.1462853
Neu1 deficiency and fibrotic lymph node microenvironment lead to imbalance in M1/M2 macrophage polarization
Abstract
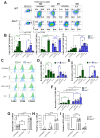
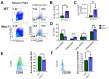
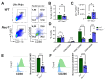
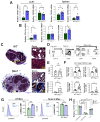
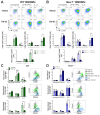
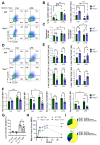
Macrophages play a pivotal role in tissue homeostasis, pathogen defense, and inflammation resolution. M1 and M2 macrophage phenotypes represent two faces in a spectrum of responses to microenvironmental changes, crucial in both physiological and pathological conditions. Neuraminidase 1 (Neu1), a lysosomal and cell surface sialidase responsible for removing terminal sialic acid residues from glycoconjugates, modulates several macrophage functions, including phagocytosis and Toll-like receptor (TLR) signaling. Current evidence suggests that Neu1 expression influences M1/M2 macrophage phenotype alterations in the context of cardiovascular diseases, indicating a potential role for Neu1 in macrophage polarization. For this reason, we investigated the impact of Neu1 deficiency on macrophage polarization in vitro and in vivo. Using bone marrow-derived macrophages (BMDMs) and peritoneal macrophages from Neu1 knockout (Neu1-/- ) mice and wild-type (WT) littermate controls, we demonstrated that Neu1-deficient macrophages exhibit an aberrant M2-like phenotype, characterized by elevated macrophage mannose receptor 1 (MMR/CD206) expression and reduced responsiveness to M1 stimuli. This M2-like phenotype was also observed in vivo in peritoneal and splenic macrophages. However, lymph node (LN) macrophages from Neu1-/- mice exhibited phenotypic alterations with reduced CD206 expression. Further analysis revealed that peripheral LNs from Neu1-/- mice were highly fibrotic, with overexpression of transforming growth factor-beta 1 (TGF-β1) and hyperactivated TGF-β signaling in LN macrophages. Consistently, TGF-β1 was found to alter M1/M2 macrophage polarization in vitro. Our findings showed that Neu1 deficiency prompts macrophages towards an M2 phenotype and that microenvironmental changes, particularly increased TGF-β1 in fibrotic tissues such as peripheral LNs in Neu1-/- mice, further influence M1/M2 macrophage polarization, highlighting its sensitivity to the local microenvironment. Therapeutic interventions targeting Neu1 or TGF-β signaling pathways may offer the potential to regulate macrophage behavior across different diseases.
Keywords: TGF-β; fibrosis; lymph node microenvironment; macrophage polarization; neuraminidase 1; sialidosis.
Copyright © 2024 Escalona, Olate-Briones, Albornoz-Muñoz, Bonacic-Doric, Rodríguez-Arriaza, Herrada and Escobedo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

