Cutting edge of immune response and immunosuppressants in allogeneic and xenogeneic islet transplantation
- PMID: 39346923
- PMCID: PMC11427288
- DOI: 10.3389/fimmu.2024.1455691
Cutting edge of immune response and immunosuppressants in allogeneic and xenogeneic islet transplantation
Abstract
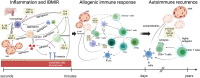
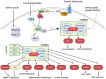
As an effective treatment for diabetes, islet transplantation has garnered significant attention and research in recent years. However, immune rejection and the toxicity of immunosuppressive drugs remain critical factors influencing the success of islet transplantation. While immunosuppressants are essential in reducing immune rejection reactions and can significantly improve the survival rate of islet transplants, improper use of these drugs can markedly increase mortality rates following transplantation. Additionally, the current availability of islet organ donations fails to meet the demand for organ transplants, making xenotransplantation a crucial method for addressing organ shortages. This review will cover the following three aspects: 1) the immune responses occurring during allogeneic islet transplantation, including three stages: inflammation and IBMIR, allogeneic immune response, and autoimmune recurrence; 2) commonly used immunosuppressants in allogeneic islet transplantation, including calcineurin inhibitors (Cyclosporine A, Tacrolimus), mycophenolate mofetil, glucocorticoids, and Bortezomib; and 3) early and late immune responses in xenogeneic islet transplantation and the immune effects of triple therapy (ECDI-fixed donor spleen cells (ECDI-SP) + anti-CD20 + Sirolimus) on xenotransplantation.
Keywords: allogenic and xenogenic islet transplantation; immune response; immunosuppressants; islet transplantation; xenotransplantation.
Copyright © 2024 Yue, Li, Yao, Song, Zhang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Chen QD, Liu L, Zhao XH, Liang JB, Li SW. Challenges and opportunities in the islet transplantation microenvironment: a comprehensive summary of inflammatory cytokine, immune cells, and vascular endothelial cells. Front Immunol. (2023) 14:1293762. doi: 10.3389/fimmu.2023.1293762 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

