Longitudinal surveillance of Coxiella burnetii following an abortion storm in domestic goats
- PMID: 39346957
- PMCID: PMC11427434
- DOI: 10.3389/fvets.2024.1426573
Longitudinal surveillance of Coxiella burnetii following an abortion storm in domestic goats
Abstract
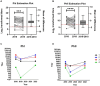
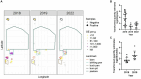
Q fever is a disease caused by Coxiella burnetii, which can cause serious illness in humans and abortions in goats. A Q fever outbreak among an unvaccinated goat herd led to a 65% loss of the kid crop in spring 2018. To assess the impact of the outbreak on the herd and environment, longitudinal surveillance of the ranch was conducted across three samplings in September 2018, April 2019, and May 2022. Antibodies against C. burnetii were monitored by an indirect immunofluorescence assay. Shedding was monitored through analysis of vaginal/fecal swabs and milk. Environmental swabs and bulk soil were collected from various locations around the ranch. Animal and environmental samples were analyzed for C. burnetii DNA by PCR. Herd-level seroprevalence decreased from 89% in 2018 to 84.3% in 2019, and 64.5% in 2022. Overall herd shedding was 14.4% in 2018, 7.4% in 2019, and 6.7% in 2022. The percentage of C. burnetii-positive environmental samples was 83.7% in 2018, 51.7% in 2019, and 28.6% in 2022. Serological evidence suggests that new infections were occurring in the herd 4 years post-abortion storm. This study demonstrates the presence of C. burnetii shedding and environmental contamination in a goat operation at least four kidding seasons after an outbreak. A better understanding of management practices that can improve outcomes for infected herds, particularly in areas without access to vaccines against C. burnetii, is needed to better protect operators and the public.
Keywords: Q fever; coxiellosis; livestock; one health; zoonosis.
Copyright © 2024 Miller, Priestley, Smith, Cherry and Kersh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Tibary A. "Abortion in Goats", in: The Merck Veterinary Manual. Rahway, NJ: Merck & Co., Inc. (2021).
LinkOut - more resources
Full Text Sources
Miscellaneous

