Coagulation depth estimation using a line scanner for depth-resolved laser speckle contrast imaging
- PMID: 39347002
- PMCID: PMC11427187
- DOI: 10.1364/BOE.529043
Coagulation depth estimation using a line scanner for depth-resolved laser speckle contrast imaging
Abstract
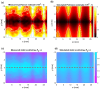
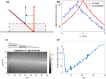
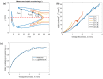
Partial-thickness burn wounds extend partially through the dermis, leaving many pain receptors intact and making the injuries very painful. Due to the painfulness, quick assessment of the burn depth is important to not delay surgery of the wound if needed. Laser speckle imaging (LSI) of skin blood flow can be helpful in finding severe coagulation zones with impaired blood flow. However, LSI measurements are typically too superficial to properly reach the full depth of the adult dermis and cannot resolve the flow in depth. Diffuse correlation spectroscopy (DCS) uses varying source-detector separations to allow differentiation of flow depths but requires time-consuming 2D scanning to form an image of the burn area. We here present a prototype for a hybrid DCS and LSI technique called speckle contrast diffuse correlation spectroscopy (scDCS) with the novel approach of using a laser line as a source and using the speckle contrast of averaged images to obtain an estimate of static scattering in the tissue. This will allow for fast non-contact 1D scanning to perform 3D tomographic imaging, making quantitative estimates of the depth and area of the coagulation zone from burn wounds. Simulations and experimental results from a volumetric flow phantom and a gelatin wedge phantom show promise to determine coagulation depth. The aim is to develop a method that, in the future, could provide more quantitative estimates of coagulation depth in partial thickness burn wounds to better estimate when surgery is needed.
© 2024 Optica Publishing Group.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Update of
- doi: 10.1364/opticaopen.25721565.
Similar articles
-
Utility of spatial frequency domain imaging (SFDI) and laser speckle imaging (LSI) to non-invasively diagnose burn depth in a porcine model.Burns. 2015 Sep;41(6):1242-52. doi: 10.1016/j.burns.2015.03.001. Epub 2015 Jun 30. Burns. 2015. PMID: 26138371 Free PMC article.
-
Quantitative long-term measurements of burns in a rat model using Spatial Frequency Domain Imaging (SFDI) and Laser Speckle Imaging (LSI).Lasers Surg Med. 2017 Mar;49(3):293-304. doi: 10.1002/lsm.22647. Epub 2017 Feb 21. Lasers Surg Med. 2017. PMID: 28220508 Free PMC article.
-
Acute discrimination between superficial-partial and deep-partial thickness burns in a preclinical model with laser speckle imaging.Burns. 2015 Aug;41(5):1058-63. doi: 10.1016/j.burns.2014.11.018. Epub 2015 Mar 24. Burns. 2015. PMID: 25814299 Free PMC article.
-
Advances in laser speckle imaging: From qualitative to quantitative hemodynamic assessment.J Biophotonics. 2024 Jan;17(1):e202300126. doi: 10.1002/jbio.202300126. Epub 2023 Oct 3. J Biophotonics. 2024. PMID: 37545037 Review.
-
Evidence Based Burn Depth Assessment Using Laser-Based Technologies: Where Do We Stand?J Burn Care Res. 2021 May 7;42(3):513-525. doi: 10.1093/jbcr/iraa195. J Burn Care Res. 2021. PMID: 33128377
Cited by
-
Diffuse Optical Spectroscopy: Technology and Applications: introduction to the feature issue.Biomed Opt Express. 2024 Oct 24;15(11):6516-6520. doi: 10.1364/BOE.542635. eCollection 2024 Nov 1. Biomed Opt Express. 2024. PMID: 39553860 Free PMC article.
References
LinkOut - more resources
Full Text Sources
