The Efficacy and Safety of Probiotic Combinations Lobun Forte® Versus Renadyl® in Patients With Chronic Kidney Disease: A Comparative, Phase IV, Randomized, Open-Label, Active-Controlled, Parallel Study
- PMID: 39347194
- PMCID: PMC11427930
- DOI: 10.7759/cureus.67987
The Efficacy and Safety of Probiotic Combinations Lobun Forte® Versus Renadyl® in Patients With Chronic Kidney Disease: A Comparative, Phase IV, Randomized, Open-Label, Active-Controlled, Parallel Study
Abstract
Background: Chronic kidney disease (CKD) often leads to gut microbiota imbalance, accelerating disease progression and increasing uremic toxins and inflammation. We conducted a randomized clinical trial comparing outcomes between two multi-strain probiotic supplements Lobun Forte® (Sanzyme P Ltd, Hyderabad, India) containing Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacterium longum, and Bacillus coagulans and Renadyl® (Kibow Biotech, LLC., Pennsylvania, United States) containing S. thermophilus, L. acidophilus, and B. longum.
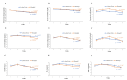
Materials and methods: Sixty patients with stage 3-4 CKD were randomized to receive either Lobun Forte (n=30) or Renadyl (n=30) for six months, with each supplement providing 45 billion CFU/capsule, twice daily. Primary outcomes included quality of life (QoL) (Short-Form 8 (SF-8) score), reductions in uremic toxins (p-cresyl sulfate (PCS), 3-indoxyl sulfate (IS), indole-3-acetic acid (IAA)), blood urea nitrogen (BUN), serum creatinine, and serum uric acid. Secondary outcomes assessed oxidative stress, inflammatory biomarkers, and estimated glomerular filtration rate (eGFR). Results: Both Lobun Forte and Renadyl groups showed significant improvements in QoL, with Lobun Forte achieving a 53.5% improvement (16.43 point increase) and Renadyl a 51.1% improvement (15.27 point increase) in SF-8 scores (p < 0.0001). The levels of IS decreased significantly in both groups (p < 0.0001), with Lobun Forte reducing IS by 29.72% and Renadyl by 24.20%. In terms of other uremic toxins, Lobun Forte showed non-significant (p > 0.05) reductions in mean PCS (7.63%) and IAA (15.57%), whereas Renadyl demonstrated a significant (p = 0.0314) decrease in PCS (20.75%) and a non-significant (p > 0.05) reduction in IAA (12.35%). Both groups showed significant (p < 0.0001) reductions in BUN and serum creatinine levels. Serum uric acid levels showed a significant (p = 0.0448) reduction with Lobun Forte while Renadyl exhibited a non-significant reduction (p = 0.1034). Lobun Forte significantly (p = 0.0359) reduced mean high-sensitivity C-reactive protein (hsCRP) levels, while Renadyl showed a non-significant reduction (p = 0.0876). Both groups had non-significant reductions in interleukin-6 and tumor necrosis factor-alpha levels (p > 0.05). Further, both groups experienced significant (p < 0.0001) increases in mean glutathione levels and nitric oxide levels. Additionally, Renadyl resulted in a significant reduction in mean malondialdehyde, whereas Lobun Forte showed a non-significant reduction. Both probiotics significantly (p < 0.0001) improved eGFR, with Lobun Forte increasing it by 40.4% and Renadyl by 36.9%. Both probiotics were well tolerated, with a favorable safety profile throughout the study. Conclusion: Both Lobun Forte and Renadyl effectively improve the quality of life in patients with stage 3-4 CKD by modulation of uremic toxins, renal parameters, inflammatory biomarkers, oxidative biomarkers, and eGFR. These findings suggest that both probiotics may help delay CKD progression by modulating the gut-kidney axis.
Keywords: chronic kidney disease (ckd); malnutrition; multi-strain probiotic supplement; quality of life (qol); uremic toxins.
Copyright © 2024, Kalidindi et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Institutional Ethics Committee, Nizam's Institute of Medical Sciences issued approval EC/NIMS/2377/2019 dated August 24, 2019. The study was prospectively registered with the Clinical Trials Registry India (CTRI/2019/10/021546) and was conducted in accordance with good clinical practice (GCP), Declaration of Helsinki. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: The study was sponsored by Sanzyme P Ltd. Financial relationships: Kishan PV and Prasad Kompella declare(s) employment from Sanzyme Pvt. Ltd, Hyderabad. KPV and PK are employees of Sanzyme Pvt. Ltd, Hyderabad, India. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Review on uremic toxins: classification, concentration, and interindividual variability. Vanholder R, De Smet R, Glorieux G, et al. Kidney Int. 2003;63:1934–1943. - PubMed
-
- Chronic kidney disease alters intestinal microbial flora. Vaziri ND, Wong J, Pahl M, et al. Kidney Int. 2013;83:308–315. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
