Engineering a Surfactant Trap via Postassembly Modification of an Imine Cage
- PMID: 39347472
- PMCID: PMC11428146
- DOI: 10.1021/acs.chemmater.4c01808
Engineering a Surfactant Trap via Postassembly Modification of an Imine Cage
Abstract
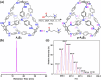
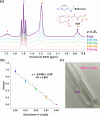
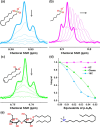
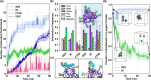
Imine self-assembly stands as a potent strategy for the preparation of molecular organic cages. However, challenges persist, such as water insolubility and limited recognition properties due to constraints in the application of specific components during the self-assembly process. In this study, we addressed these limitations by initially employing a locking strategy, followed by a postassembly modification. This sequential approach enables precise control over both the solubility and host-guest properties of an imine-based cage. The resulting structure demonstrates water solubility and exhibits an exceptional capacity to selectively interact with anionic surfactants, inducing their precipitation. Remarkably, each cage precipitates 24 equiv of anionic surfactants even at concentrations much lower than the surfactant's critical micelle concentration (CMC), ensuring their complete removal. Molecular simulations elucidate how anionic surfactants specifically interact with the cage to facilitate aggregation below the surfactant CMC and induce precipitation as a micellar cross-linker. This innovative class of cages paves the way for the advancement of materials tailored for environmental remediation.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): J.M., A.C., and M.P-F. are the inventors of a pending Spanish patent application.
Figures


References
LinkOut - more resources
Full Text Sources
